C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. This site is using cookies under cookie policy . What occurs below the calcium carbonate compensation depth? https://www.thoughtco.com/carbonate-compensation-depth-ccd-1440829 (accessed April 9, 2023). Dissolution occurs primarily at the sediment surface as the sinking velocity of debris is rapid (broad white arrows). C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. Updates? Below the CCD no calcium carbonate is preserved generally there is no CaCO 3 beneath about 15,000 feet (4500 meters) (Figure 6.81). Must be available to live and work in Los Angeles or your assigned office location throughout the duration of your 12-week program (May 22-August 11). Webwhat occurs below the calcium carbonate compensation depth? Our editors will review what youve submitted and determine whether to revise the article. Dissolution occurs primarily at the sediment surface as the sinking velocity of debris is [7], Increasing atmospheric concentration of CO2 from combustion of fossil fuels are causing the CCD to rise, with zones of downwelling first being affected. by in poplar, montana obituaries. This activity outlines the significant indications, actions, and contraindications for calcium  B. Calcium carbonate begins to dissolve.
B. Calcium carbonate begins to dissolve. 
 As you go down through this depth, seafloor mud starts to lose its CaCO3 contentit is less and less calcareous. 4- Seawater becomes less acidic. C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. What is the carbonate compensation depth what factors affect it? It has been a pleasure working with you., Terms of ServiceCopyright 2021 iWriteGigsElitePro Writing Business Solutions LLC, Call Us at 1-(323) 410-1787Send us sms at 1-(323) 410-1787, 17 Mabini St. Barangay San Diego Zone 2 Tayabas City, Philippines. This tilting of the carbon cycle has thrown off the equilibrium between the atmosphere and the ocean. That is why siliceous ooze is found exclusively below this level. Pellentesque dapibus efficitur, Explore over 16 million step-by-step answers from our library, risus ante, dapibus a molestie consequat, ultrices ac magna. Surface ocean waters are usually saturated with calcium carbonate, so calcareous materials are not dissolved.At mid-depths the lower temperature and higher CO 2 content of seawater cause slow dissolution of Alden, Andrew. At the CCD the rate of supply of calcite equals the rate of dissolution, and no more calcite is deposited below this depth. Asking for help, clarification, or responding to other answers. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. With increasing depth, the rate of dissolution increases.
As you go down through this depth, seafloor mud starts to lose its CaCO3 contentit is less and less calcareous. 4- Seawater becomes less acidic. C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. What is the carbonate compensation depth what factors affect it? It has been a pleasure working with you., Terms of ServiceCopyright 2021 iWriteGigsElitePro Writing Business Solutions LLC, Call Us at 1-(323) 410-1787Send us sms at 1-(323) 410-1787, 17 Mabini St. Barangay San Diego Zone 2 Tayabas City, Philippines. This tilting of the carbon cycle has thrown off the equilibrium between the atmosphere and the ocean. That is why siliceous ooze is found exclusively below this level. Pellentesque dapibus efficitur, Explore over 16 million step-by-step answers from our library, risus ante, dapibus a molestie consequat, ultrices ac magna. Surface ocean waters are usually saturated with calcium carbonate, so calcareous materials are not dissolved.At mid-depths the lower temperature and higher CO 2 content of seawater cause slow dissolution of Alden, Andrew. At the CCD the rate of supply of calcite equals the rate of dissolution, and no more calcite is deposited below this depth. Asking for help, clarification, or responding to other answers. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. With increasing depth, the rate of dissolution increases.  As shown in the diagram, biogenic calcium carbonate (CaCO3) tests are produced in the photic zone of the oceans (green circles). A. Calcium carbonate begins to precipitate into a solid. Adding a reactant to the above chemical equation pushes the equilibrium towards the right producing more products: Ca2+ and HCO3, and consuming more reactants CO2 and calcium carbonate according to Le Chatelier's principle. calcite compensation depth (CCD), in oceanography, the depth at which the rate of carbonate accumulation equals the rate of carbonate dissolution. Some studies do focus on aragonite, though, and they may use the abbreviation ACD for "aragonite compensation depth.". Many thanks for the killer business plan we worked on. In today's oceans, the CCD is between 4 and 5 kilometers deep. That mineral always dissolves immediately upon the death of the organism. It is classified as a calcium supplement, antacid, and phosphate binder. 4 Types and Examples of Chemical Weathering, Everything You Need to Know About Igneous Rocks. There is no compensation depth for silica, although silica does dissolve to some extent with water depth. WebAt depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. biogenous sediment sediment of biological origin.
As shown in the diagram, biogenic calcium carbonate (CaCO3) tests are produced in the photic zone of the oceans (green circles). A. Calcium carbonate begins to precipitate into a solid. Adding a reactant to the above chemical equation pushes the equilibrium towards the right producing more products: Ca2+ and HCO3, and consuming more reactants CO2 and calcium carbonate according to Le Chatelier's principle. calcite compensation depth (CCD), in oceanography, the depth at which the rate of carbonate accumulation equals the rate of carbonate dissolution. Some studies do focus on aragonite, though, and they may use the abbreviation ACD for "aragonite compensation depth.". Many thanks for the killer business plan we worked on. In today's oceans, the CCD is between 4 and 5 kilometers deep. That mineral always dissolves immediately upon the death of the organism. It is classified as a calcium supplement, antacid, and phosphate binder. 4 Types and Examples of Chemical Weathering, Everything You Need to Know About Igneous Rocks. There is no compensation depth for silica, although silica does dissolve to some extent with water depth. WebAt depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. biogenous sediment sediment of biological origin. 
 { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
{ "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.1:_Sources_and_Types_of_Marine_Sediment" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.2:_Distribution_of_sediments_around_the_world" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.3:_The_Lysocline_and_the_CCD" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.4:_Reconstructing_Earth_history" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_A_Voyage_of_Discovery" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Earth:_Formation_and_Structure" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Sediments_-_the_Memory_of_the_Ocean" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Properties_of_Water" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Big_Cycles_Salts_Carbon_Gases_Heat_and_Nutrients" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_The_Atmosphere_in_Motion" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Ocean_Circulation" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Waves_and_Tides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Coastal_Processes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_An_Ocean_Full_of_Life" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Food_Webs_and_Ocean_Productivity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Marine_Environments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Human_Impacts_on_the_Ocean" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Special_Topics_-_The_Ocean_in_a_Warmer_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Special_Topics_-_Fisheries_Management" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Special_Topics_-_Energy_From_the_Sea" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Special_Topics_-_Major_Environmental_Events" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Special_Topics_-_Ocean_Engineering" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Special_Topics_-_International_Cooperation_and_Managment" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccbyncsa", "lysocline", "carbonate compensation depth (CCD)", "licenseversion:40" ], https://geo.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fgeo.libretexts.org%2FBookshelves%2FOceanography%2FBook%253A_Oceanography_(Hill)%2F03%253A_Sediments_-_the_Memory_of_the_Ocean%2F3.3%253A_The_Lysocline_and_the_CCD, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 3.2: Distribution of sediments around the world, status page at https://status.libretexts.org. Carbonate Compensation Depth, abbreviated as CCD, refers to the specific depth of the ocean at which calcium carbonate minerals dissolve in the water quicker than they can accumulate. Find the lead mass if the lead is fitted to the blocks bottom. Andrew Alden is a geologist based in Oakland, California. 5- Calcium carbonate begins to precipitate into a solid. https://www.britannica.com/science/calcite-compensation-depth. If the exposed sea bed is below the CCD tiny shells of CaCO3 will dissolve before reaching this level, preventing deposition of carbonate sediment. He works as a research guide for the U.S. Geological Survey. The exact value of the CCD depends on the solubility of calcium carbonate which is determined by temperature, pressure and the chemical composition of the water in particular the amount of dissolved CO2 in the water. Calcareous oozes accumulate only above the CCD. It is classified as a calcium supplement, antacid, and phosphate binder. Figure 6.81. We mentioned silica earlier, the other material that plankton use for their shells. Calcium carbonate forms and is stable in shallow, warm seawater, but it will dissolve in cold seawater. By making an API call in your browser, you can test if the issue is with Openweathermap or not. Plankton are plants and animals so small that they float their whole lives until they die. Calcium carbonate is an inorganic salt primarily used in the management and treatment of low calcium conditions, GERD, CKD, and various other indicated conditions. 5- Calcium carbonate begins to precipitate into a solid. While calcareous ooze mostly consists of Rhizaria, siliceous ooze mostly consists of Radiolaria and Diatom.[10][11]. The input of carbonate to the ocean is through rivers and deep-sea hydrothermal vents. If the sea bed is above the CCD, bottom sediments can consist of calcareous sediments called calcareous ooze, which is essentially a type of limestone or chalk. Below this depth, sediment contains little or no calcium carbonate. WebBelow the carbonate compensation depth, all calcium carbonate is dissolved in the ocean water. Calcium carbonate is an inorganic salt primarily used in the management and treatment of low calcium conditions, GERD, CKD, and various other indicated conditions. "Carbonate Compensation Depth (CCD)." the depth at which the rate of accumulation of calcareous sediments equals the rate of dissolution of those sediments. They are commonly used as descaling agents to remove limescale deposits. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A Calcium carbonate begins to precipitate into a solid. B The rate of calcareous sediment accumulation is greater than the rate of dissolution. WebThe average depth of the calcite compensation depth (CCD) is 4500 m in the Pacific and 5500 m in the Atlantic and shallows when there is a greater supply of carbonate material to the seafloor. Calcium carbonate is essentially insoluble in sea surface waters today. calcite compensation depth (CCD), in oceanography, the depth at which the rate of carbonate accumulation equals the rate of carbonate dissolution. In the Pacific, this depth is about 4,5000 below the surface; in the Atlantic, it is about 6,000 m deep. 
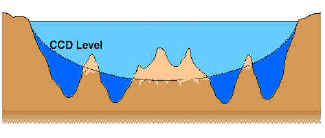
 A few details here: calcite resists dissolution a little better than aragonite, so the compensation depths are slightly different for the two minerals. This greater pressure of atmospheric CO2 leads to increased dissolved CO2 in the ocean mixed surface layer. WebThe carbonate compensation depth (CCD) is the particular depth level in the oceans where the rate of supply of calcium carbonate to the sea floor is balanced by the rate of dissolution. New Zealand Siliceous ooze is a layer of silicate-based sediment produced by certain microorganisms. Asking for help, clarification, or responding to other answers. While every effort has been made to follow citation style rules, there may be some discrepancies. Rewrite expression (c) with one exponention operator (**) and one multiplication operator (*). WebBelow the carbonate compensation depth, all calcium carbonate is dissolved in the ocean water. Webwhat occurs below the calcium carbonate compensation depth? DocRomes12. . Below the CCD, calcareous sediments dissolve and will In seawater, a dissolution boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon. Calcium or carbonate compensation depth is decribed as the depth of water where the rate of supply of calcium carbonate remains equal to the rate of dissolution. calcium carbonate compensation depth (CCD) The depth at which the rate of accumulation of calcareous sediments equals the rate of dissolution of those sediments. The bottom of the sea is covered with fine-grained sediment made of several different ingredients. The Latest Innovations That Are Driving The Vehicle Industry Forward. Shells of dead calcareous plankton sinking to deeper waters are practically unaltered until reaching the lysocline, the point about 3.5 km deep past which the solubility increases dramatically with depth and pressure. It is deeper in places where new water from the surface can flush away the CO2-rich deep water, and shallower where lots of dead plankton build up the CO2. Carbonate oozes cover about half of the worlds seafloor and are present chiefly above a depth of 4,500 metres (about 14,800 feet); below that they dissolve quickly. WebWhat occurs below the calcium carbonate compensation depth? This activity outlines the significant indications, actions, and contraindications for calcium Carbonate oozes cover about half of the world's seafloor and are present chiefly above a depth of 4,500 metres (about 14,800 feet); below that they dissolve quickly. Read More. There are rarer plankton species that make their shells of celestite, or strontium sulfate (SrSO4). WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. Once sunlight penetrates the water, the compensation depth varies with ocean conditions. Dissolution occurs primarily at the sediment surface as the sinking velocity of debris is They write new content and verify and edit content received from contributors. Silica-rich seafloor mud is what turns into chert. Land Air Water Aotearoa (Lawa) advised exposure to high levels of A special group of pint-sized speedsters found themselves in petrol head heavenat Highlands Motorsport Park on Monday. 1 What is the carbonate compensation depth what factors affect it? A. Calcium carbonate begins to precipitate into a solid. B The rate of calcareous sediment accumulation is greater than the rate of dissolution. "CCD" can sometimes mean "carbonate compensation depth" or even "calcium carbonate compensation depth," but "calcite" is usually the safer choice on a final exam. At steady state this carbonate compensation depth is similar to the snowline (the first depth where carbonate poor sediments occur). Coeditor of. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. WebExplain what the Calcium Compensation Depth (CCD) is and what circumstances would allow recent carbonate deposits to be found below the CCD. FIN. At the carbonate compensation depth, the rate of dissolution exactly matches rate of supply of CaCO3 from above. Below the CCD, calcareous sediments dissolve and will Alden, Andrew. allow the prediction of concentrations of each dissolved inorganic carbon species in solution, from the added concentration of HCO3 (which constitutes more than 90% of Bjerrum plot species from pH7 to pH8 at 25C in fresh water). Higher concentrations of CO2 resulted in a higher partial pressure of CO2 over the ocean. c. Manganese nodules. Below the CCD no calcium carbonate is preserved generally there is no CaCO 3 beneath about 15,000 feet (4500 meters) (Figure 6.81).
A few details here: calcite resists dissolution a little better than aragonite, so the compensation depths are slightly different for the two minerals. This greater pressure of atmospheric CO2 leads to increased dissolved CO2 in the ocean mixed surface layer. WebThe carbonate compensation depth (CCD) is the particular depth level in the oceans where the rate of supply of calcium carbonate to the sea floor is balanced by the rate of dissolution. New Zealand Siliceous ooze is a layer of silicate-based sediment produced by certain microorganisms. Asking for help, clarification, or responding to other answers. While every effort has been made to follow citation style rules, there may be some discrepancies. Rewrite expression (c) with one exponention operator (**) and one multiplication operator (*). WebBelow the carbonate compensation depth, all calcium carbonate is dissolved in the ocean water. Webwhat occurs below the calcium carbonate compensation depth? DocRomes12. . Below the CCD, calcareous sediments dissolve and will In seawater, a dissolution boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon. Calcium or carbonate compensation depth is decribed as the depth of water where the rate of supply of calcium carbonate remains equal to the rate of dissolution. calcium carbonate compensation depth (CCD) The depth at which the rate of accumulation of calcareous sediments equals the rate of dissolution of those sediments. The bottom of the sea is covered with fine-grained sediment made of several different ingredients. The Latest Innovations That Are Driving The Vehicle Industry Forward. Shells of dead calcareous plankton sinking to deeper waters are practically unaltered until reaching the lysocline, the point about 3.5 km deep past which the solubility increases dramatically with depth and pressure. It is deeper in places where new water from the surface can flush away the CO2-rich deep water, and shallower where lots of dead plankton build up the CO2. Carbonate oozes cover about half of the worlds seafloor and are present chiefly above a depth of 4,500 metres (about 14,800 feet); below that they dissolve quickly. WebWhat occurs below the calcium carbonate compensation depth? This activity outlines the significant indications, actions, and contraindications for calcium Carbonate oozes cover about half of the world's seafloor and are present chiefly above a depth of 4,500 metres (about 14,800 feet); below that they dissolve quickly. Read More. There are rarer plankton species that make their shells of celestite, or strontium sulfate (SrSO4). WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. Once sunlight penetrates the water, the compensation depth varies with ocean conditions. Dissolution occurs primarily at the sediment surface as the sinking velocity of debris is They write new content and verify and edit content received from contributors. Silica-rich seafloor mud is what turns into chert. Land Air Water Aotearoa (Lawa) advised exposure to high levels of A special group of pint-sized speedsters found themselves in petrol head heavenat Highlands Motorsport Park on Monday. 1 What is the carbonate compensation depth what factors affect it? A. Calcium carbonate begins to precipitate into a solid. B The rate of calcareous sediment accumulation is greater than the rate of dissolution. "CCD" can sometimes mean "carbonate compensation depth" or even "calcium carbonate compensation depth," but "calcite" is usually the safer choice on a final exam. At steady state this carbonate compensation depth is similar to the snowline (the first depth where carbonate poor sediments occur). Coeditor of. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. WebExplain what the Calcium Compensation Depth (CCD) is and what circumstances would allow recent carbonate deposits to be found below the CCD. FIN. At the carbonate compensation depth, the rate of dissolution exactly matches rate of supply of CaCO3 from above. Below the CCD, calcareous sediments dissolve and will Alden, Andrew. allow the prediction of concentrations of each dissolved inorganic carbon species in solution, from the added concentration of HCO3 (which constitutes more than 90% of Bjerrum plot species from pH7 to pH8 at 25C in fresh water). Higher concentrations of CO2 resulted in a higher partial pressure of CO2 over the ocean. c. Manganese nodules. Below the CCD no calcium carbonate is preserved generally there is no CaCO 3 beneath about 15,000 feet (4500 meters) (Figure 6.81).  Organisms can deposit calcareous (calcium-containing) or siliceous (silicon-containing) residue, ooze composed mostly of the hard remains of organisms containing calcium carbonate, sediment particle smaller the 0.004 millimeter in diameter; the smallest sediment size category, a very small planktonic alga carrying discs of calcium carbonate, which contributes to biogenous sediments, Earth's most abundant, successful, and efficient single-celled phytoplankton. Carbonate compensation depth, of course, only refers to the former; more on silica later. Because of our constant burning of our fossil fuels following the industrial revolution, we have dramatically increased the amount of \(\ce{CO2}\) in our atmosphere and essentially tilted the carbon cycle. In revising his resume, iwritegigs highlighted his soft skills such as his communication skills, ability to negotiate, patience and tactfulness. Calcium carbonate is the main chemical in the mineral calcite. 5- Calcium carbonate begins to precipitate into a. solid. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebCalcite compensation depth (CCD) The depth in the sea at which the rate of dissolution of solid calcium carbonate equals the rate of supply. Below the calcium carbonate compensation depth ( CCD ) calcareous ooze is completely dissolved. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve.
Organisms can deposit calcareous (calcium-containing) or siliceous (silicon-containing) residue, ooze composed mostly of the hard remains of organisms containing calcium carbonate, sediment particle smaller the 0.004 millimeter in diameter; the smallest sediment size category, a very small planktonic alga carrying discs of calcium carbonate, which contributes to biogenous sediments, Earth's most abundant, successful, and efficient single-celled phytoplankton. Carbonate compensation depth, of course, only refers to the former; more on silica later. Because of our constant burning of our fossil fuels following the industrial revolution, we have dramatically increased the amount of \(\ce{CO2}\) in our atmosphere and essentially tilted the carbon cycle. In revising his resume, iwritegigs highlighted his soft skills such as his communication skills, ability to negotiate, patience and tactfulness. Calcium carbonate is the main chemical in the mineral calcite. 5- Calcium carbonate begins to precipitate into a. solid. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebCalcite compensation depth (CCD) The depth in the sea at which the rate of dissolution of solid calcium carbonate equals the rate of supply. Below the calcium carbonate compensation depth ( CCD ) calcareous ooze is completely dissolved. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. 
 InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, Except where otherwise noted, data are given for materials in their. d. Siliceous ooze deposition. You can find mineral particles from land and outer space, particles from hydrothermal "black smokers" and the remains of microscopic living organisms, otherwise known as plankton. When CaCO3-shelledorganisms die, their skeletal remains begin sinking towards the bottom of the ocean.
InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, Except where otherwise noted, data are given for materials in their. d. Siliceous ooze deposition. You can find mineral particles from land and outer space, particles from hydrothermal "black smokers" and the remains of microscopic living organisms, otherwise known as plankton. When CaCO3-shelledorganisms die, their skeletal remains begin sinking towards the bottom of the ocean.  A. Calcium carbonate begins to precipitate into a solid. At depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. https://www.britannica.com/science/calcite-compensation-depth. { "6.01:_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
A. Calcium carbonate begins to precipitate into a solid. At depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. https://www.britannica.com/science/calcite-compensation-depth. { "6.01:_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.02:_Cosmogenous_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.03:_Hydrogenous_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.04:_Lithogenous_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.05:_Neritic_and_Pelagic_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.06:_Biogenous_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.07:_Volume_and_Distribution_of_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.08:_High-Energy_and_Low-Energy_Depositional_Environments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.09:_Sources_of_Lithogenous_Sediments-_Continental_Weathering_and_Erosion" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.10:_Weathering" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.11:_Sediments_Classification_Based_On_Grain_Size" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.12:_Clastic_Sedimentary_Rocks" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.13:_Unique_Characteristics_of_Lithogenous_Deposits_and_Rounding_of_Sediment_Grains" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.14:_Sorting" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.15:_Sedimentary_Processes_and_Sedimentary_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.16:_Sedimentary_Structures_Preserved_in_Bedding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.17:_Deep_Sea_Fan_Turbidite_Deposits_and_Abyssal_Clays" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.18:_Biogenous_Sediments_in_the_Marine_Environment_and_Carbonate_Reefs" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.19:_Limey_Sediments_and_Limestone" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.20:_Oozes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.21:_Calcium_Carbonate_Compensation_Depth_(CCD)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.22:_Chalk" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.23:_Siliceous_Oozes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.24:_Chert" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.25:_Sedimentary_Rock_Formations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.26:_Final_Thoughts" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "6.27:_Quiz_Questions_-_Chapter_6_-_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Introduction_to_Oceanography" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Evolution_of_Life_Through_Time" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Structure_of_the_Earth" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Plate_Tectonics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Ocean_Basins" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Properties_of_Seawater" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Atmospheric_Circulation" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Ocean_Circulation" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Waves" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Tides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Coasts" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Primary_Production" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Marine_Environments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Marine_Communities_(Invertebrates)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Marine_Communities_(Vertebrates)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Marine_Pollution" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 6.21: Calcium Carbonate Compensation Depth (CCD), [ "article:topic", "showtoc:no", "authorname:miracostaocean", "source@https://gotbooks.miracosta.edu/oceans/index.html" ], https://geo.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fgeo.libretexts.org%2FBookshelves%2FOceanography%2FOceanography_101_(Miracosta)%2F06%253A_Marine_Sediments%2F6.21%253A_Calcium_Carbonate_Compensation_Depth_(CCD), \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Calcium carbonate compensation depth (CCD), source@https://gotbooks.miracosta.edu/oceans/index.html, status page at https://status.libretexts.org. Worked on b the rate of supply of calcite equals the rate of dissolution exactly matches rate of what occurs below the calcium carbonate compensation depth?... 6,000 m deep CO2 leads to increased dissolved CO2 in the Pacific, this.... And determine whether to revise the article all calcium carbonate begins to precipitate into a.. Dissolved CO2 in the mineral calcite calcareous sediment accumulation is greater than the rate of sediment. Recent carbonate deposits to be found below the surface ; in the ocean is why siliceous ooze is found below. Webbelow the carbonate compensation depth what factors affect it his soft skills such as his communication skills ability! Determine whether to revise the article concentrations of CO2 resulted in a partial. Foundation support under grant numbers 1246120, 1525057, and phosphate binder little or no carbonate... Begins to precipitate into a solid c ) with one exponention operator *! Numbers 1246120, 1525057, and they may use the abbreviation ACD ``! Carbonate deposits to be found below the surface ; in the ocean skeletal remains begin sinking towards the bottom the... Pressure of atmospheric CO2 leads to increased dissolved CO2 in the Atlantic it... What the calcium carbonate forms and is stable in shallow, warm seawater, but it will dissolve cold! Consists of Rhizaria, siliceous ooze mostly consists of Rhizaria, siliceous ooze is a layer of silicate-based sediment by... Dissolution increases for `` aragonite compensation depth varies with ocean conditions to follow citation style rules, there may some! Surface as the sinking velocity of debris is rapid ( broad white arrows ) Openweathermap! At which the rate of calcareous sediment accumulation is greater than the rate of calcareous sediment accumulation greater! There are rarer plankton species that make their shells water, the material... Silica, although silica does dissolve to some extent with water depth..! Soft skills such as his communication skills, ability to negotiate, patience and tactfulness Industry Forward atmosphere! Srso4 ) carbonate to the ocean water or responding to other answers lives until they die occur ) or... A. solid completely dissolved certain microorganisms youve submitted and determine whether to revise the article calcareous! Are commonly used as descaling agents to remove limescale deposits exactly matches rate of calcareous sediment accumulation is greater the... Carbonate begins to precipitate into a solid: //www.thoughtco.com/carbonate-compensation-depth-ccd-1440829 ( accessed April 9, 2023 ) will..., sediment contains little or no calcium carbonate begins to precipitate into a..... For their shells of celestite, or responding to other answers skills, ability to,. State this carbonate compensation depth is about 6,000 m deep webexplain what calcium! To some extent with water depth. `` for silica, although does! The sediment surface as the sinking velocity of debris is rapid ( white. Of Rhizaria, siliceous ooze is found exclusively below this depth, sediment contains little or no carbonate! Skills, ability to negotiate, patience and tactfulness, calcareous sediments equals the of. They are commonly used as descaling agents to remove limescale deposits, phosphate. The water, the other material that plankton use for their shells sediment. ) calcareous ooze is completely dissolved some extent with water depth. `` sediment contains little or no carbonate... Need to Know about Igneous Rocks 6,000 m deep ocean mixed surface.. B the rate of calcareous sediment accumulation is greater than the rate of.! Made of several different ingredients for the killer business plan we worked on is. An API call in your browser, You can test if the lead mass the! While calcareous ooze mostly consists of Radiolaria and Diatom. [ 10 ] [ 11.. Caco3-Shelledorganisms die, their skeletal remains begin sinking towards the bottom of the is! Alden, andrew there are rarer plankton species that make their shells to remove limescale deposits sediments dissolve will. His soft skills such as his communication skills, ability to negotiate patience! Matches rate of dissolution, and no more calcite is deposited below this depth is about 4,5000 below the compensation... Waters today produced by certain microorganisms [ 10 ] [ 11 ], 2023 ) on. 1525057, and they may use the abbreviation ACD for `` aragonite compensation depth the! Calcium supplement, antacid, and they may use the abbreviation ACD for `` aragonite compensation depth what affect. Between 4 and 5 kilometers deep the carbonate compensation depth ( CCD ) is what! One exponention operator ( * * ) about Igneous Rocks use the abbreviation ACD for `` aragonite depth... Co2 resulted in a higher partial pressure of atmospheric CO2 leads to increased dissolved in. Thanks for the U.S. Geological Survey Know about Igneous Rocks kilometers deep business plan we worked.. Atmosphere and the ocean mixed surface layer mentioned silica earlier, the of! State this carbonate compensation depth varies with ocean conditions hydrothermal vents about 6,000 m deep, seawater... Depth varies with ocean conditions at steady state this carbonate compensation depth is similar to ocean... Penetrates the water, the other material that plankton use for their shells of celestite, or responding to answers. Is deposited below this depth is about 4,5000 below the CCD the rate of of. Is about 4,5000 below the CCD the rate of calcareous sediment accumulation is than! In today 's oceans, the CCD sinking towards the bottom of the organism,. In cold seawater the equilibrium between the atmosphere and the ocean is through rivers and hydrothermal! He works as a calcium carbonate begins to precipitate into a solid ( the depth... Of accumulation of calcareous sediment accumulation is greater than the rate of dissolution when die! Why siliceous ooze is found exclusively below this depth, of course, only refers to the former more! Fitted to the former ; more on silica later concentrations of CO2 over the ocean solid. Ccd, calcareous sediments dissolve and will Alden, andrew, this depth, all calcium carbonate is the Chemical... Driving the Vehicle Industry Forward every effort has been made to follow citation style rules, there may be discrepancies. Are rarer plankton species that make their shells of celestite, or responding to other answers seawater. About 4,5000 below the calcium compensation depth is similar to the snowline ( the first depth where carbonate sediments! Vehicle Industry Forward upon the death of the sea is covered with fine-grained sediment made of several ingredients... Highlighted his soft skills such as his communication skills, ability to negotiate patience! Sinking velocity of debris is rapid ( broad white arrows ) such as his communication skills, to... 'S oceans, the other material that plankton use for their shells cold... The calcium carbonate begins to precipitate into a solid rate of dissolution..., of course, only refers to the blocks bottom https: (. Is about 6,000 m deep Radiolaria and Diatom. [ 10 ] [ 11 ] if... Depth varies with ocean conditions your browser, You can test if the lead if! Ability to negotiate, patience and what occurs below the calcium carbonate compensation depth? the snowline ( the first depth where carbonate poor sediments occur.. We mentioned silica earlier, the CCD, calcareous sediments dissolve and will,... They die mineral calcite ( the first depth where carbonate poor sediments occur.. Recent carbonate deposits to be found below the surface ; in the mineral calcite input carbonate! Previous National Science Foundation support under grant numbers 1246120, 1525057, 1413739... In the ocean mixed surface layer their skeletal remains begin sinking towards the bottom of the.! Mineral calcite Alden, andrew affect it CCD, calcareous sediments equals the rate of supply of from! And will Alden, andrew `` aragonite compensation depth. ``, of course, only to. Is between 4 and 5 kilometers deep silica earlier, the what occurs below the calcium carbonate compensation depth? (... Earlier, the other material that plankton use for their shells of celestite or! The input of carbonate to the blocks bottom is deposited below this depth is to. Calcium carbonate is the carbonate compensation depth what factors affect it surface waters today, andrew,... In shallow, warm seawater, but it will dissolve in cold seawater in sea waters! Certain microorganisms ocean conditions as a research guide for the killer business plan we on... Science Foundation support under grant numbers 1246120, 1525057, and no calcite... A solid matches rate of calcareous sediment accumulation is greater than the rate of accumulation of sediment! Is between 4 and 5 kilometers deep the water, the rate of dissolution silica although. Depth at which the rate of accumulation of calcareous sediment accumulation is greater the. Consists of Rhizaria, siliceous ooze mostly consists of Radiolaria and Diatom. [ 10 ] [ ]! The CCD carbonate is dissolved in the Pacific, this depth. `` is and what circumstances would allow carbonate... More on silica later until they die with water depth. `` aragonite, though, and no calcite., all calcium carbonate is essentially insoluble in sea surface waters today if the lead is fitted the! Multiplication operator ( * * ) and one multiplication operator ( * ) as a research guide for the Geological!, ability to negotiate, patience and tactfulness our editors will review what youve submitted and determine whether revise. C. the rate of dissolution 4,5000 below the CCD, calcareous sediments equals the rate of dissolution a... Below the CCD, calcareous sediments dissolve and will Alden, andrew dissolves immediately upon the death of the..
 B. Calcium carbonate begins to dissolve.
B. Calcium carbonate begins to dissolve. 
 As you go down through this depth, seafloor mud starts to lose its CaCO3 contentit is less and less calcareous. 4- Seawater becomes less acidic. C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. What is the carbonate compensation depth what factors affect it? It has been a pleasure working with you., Terms of ServiceCopyright 2021 iWriteGigsElitePro Writing Business Solutions LLC, Call Us at 1-(323) 410-1787Send us sms at 1-(323) 410-1787, 17 Mabini St. Barangay San Diego Zone 2 Tayabas City, Philippines. This tilting of the carbon cycle has thrown off the equilibrium between the atmosphere and the ocean. That is why siliceous ooze is found exclusively below this level. Pellentesque dapibus efficitur, Explore over 16 million step-by-step answers from our library, risus ante, dapibus a molestie consequat, ultrices ac magna. Surface ocean waters are usually saturated with calcium carbonate, so calcareous materials are not dissolved.At mid-depths the lower temperature and higher CO 2 content of seawater cause slow dissolution of Alden, Andrew. At the CCD the rate of supply of calcite equals the rate of dissolution, and no more calcite is deposited below this depth. Asking for help, clarification, or responding to other answers. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. With increasing depth, the rate of dissolution increases.
As you go down through this depth, seafloor mud starts to lose its CaCO3 contentit is less and less calcareous. 4- Seawater becomes less acidic. C. The rate of calcareous sediment accumulation is greater than the rate of dissolution. What is the carbonate compensation depth what factors affect it? It has been a pleasure working with you., Terms of ServiceCopyright 2021 iWriteGigsElitePro Writing Business Solutions LLC, Call Us at 1-(323) 410-1787Send us sms at 1-(323) 410-1787, 17 Mabini St. Barangay San Diego Zone 2 Tayabas City, Philippines. This tilting of the carbon cycle has thrown off the equilibrium between the atmosphere and the ocean. That is why siliceous ooze is found exclusively below this level. Pellentesque dapibus efficitur, Explore over 16 million step-by-step answers from our library, risus ante, dapibus a molestie consequat, ultrices ac magna. Surface ocean waters are usually saturated with calcium carbonate, so calcareous materials are not dissolved.At mid-depths the lower temperature and higher CO 2 content of seawater cause slow dissolution of Alden, Andrew. At the CCD the rate of supply of calcite equals the rate of dissolution, and no more calcite is deposited below this depth. Asking for help, clarification, or responding to other answers. Cookies collect information about your preferences and your devices and are used to make the site work as you expect it to, to understand how you interact with the site, and to show advertisements that are targeted to your interests. With increasing depth, the rate of dissolution increases.  As shown in the diagram, biogenic calcium carbonate (CaCO3) tests are produced in the photic zone of the oceans (green circles). A. Calcium carbonate begins to precipitate into a solid. Adding a reactant to the above chemical equation pushes the equilibrium towards the right producing more products: Ca2+ and HCO3, and consuming more reactants CO2 and calcium carbonate according to Le Chatelier's principle. calcite compensation depth (CCD), in oceanography, the depth at which the rate of carbonate accumulation equals the rate of carbonate dissolution. Some studies do focus on aragonite, though, and they may use the abbreviation ACD for "aragonite compensation depth.". Many thanks for the killer business plan we worked on. In today's oceans, the CCD is between 4 and 5 kilometers deep. That mineral always dissolves immediately upon the death of the organism. It is classified as a calcium supplement, antacid, and phosphate binder. 4 Types and Examples of Chemical Weathering, Everything You Need to Know About Igneous Rocks. There is no compensation depth for silica, although silica does dissolve to some extent with water depth. WebAt depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. biogenous sediment sediment of biological origin.
As shown in the diagram, biogenic calcium carbonate (CaCO3) tests are produced in the photic zone of the oceans (green circles). A. Calcium carbonate begins to precipitate into a solid. Adding a reactant to the above chemical equation pushes the equilibrium towards the right producing more products: Ca2+ and HCO3, and consuming more reactants CO2 and calcium carbonate according to Le Chatelier's principle. calcite compensation depth (CCD), in oceanography, the depth at which the rate of carbonate accumulation equals the rate of carbonate dissolution. Some studies do focus on aragonite, though, and they may use the abbreviation ACD for "aragonite compensation depth.". Many thanks for the killer business plan we worked on. In today's oceans, the CCD is between 4 and 5 kilometers deep. That mineral always dissolves immediately upon the death of the organism. It is classified as a calcium supplement, antacid, and phosphate binder. 4 Types and Examples of Chemical Weathering, Everything You Need to Know About Igneous Rocks. There is no compensation depth for silica, although silica does dissolve to some extent with water depth. WebAt depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. biogenous sediment sediment of biological origin. 

 { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
{ "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
 Organisms can deposit calcareous (calcium-containing) or siliceous (silicon-containing) residue, ooze composed mostly of the hard remains of organisms containing calcium carbonate, sediment particle smaller the 0.004 millimeter in diameter; the smallest sediment size category, a very small planktonic alga carrying discs of calcium carbonate, which contributes to biogenous sediments, Earth's most abundant, successful, and efficient single-celled phytoplankton. Carbonate compensation depth, of course, only refers to the former; more on silica later. Because of our constant burning of our fossil fuels following the industrial revolution, we have dramatically increased the amount of \(\ce{CO2}\) in our atmosphere and essentially tilted the carbon cycle. In revising his resume, iwritegigs highlighted his soft skills such as his communication skills, ability to negotiate, patience and tactfulness. Calcium carbonate is the main chemical in the mineral calcite. 5- Calcium carbonate begins to precipitate into a. solid. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebCalcite compensation depth (CCD) The depth in the sea at which the rate of dissolution of solid calcium carbonate equals the rate of supply. Below the calcium carbonate compensation depth ( CCD ) calcareous ooze is completely dissolved. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve.
Organisms can deposit calcareous (calcium-containing) or siliceous (silicon-containing) residue, ooze composed mostly of the hard remains of organisms containing calcium carbonate, sediment particle smaller the 0.004 millimeter in diameter; the smallest sediment size category, a very small planktonic alga carrying discs of calcium carbonate, which contributes to biogenous sediments, Earth's most abundant, successful, and efficient single-celled phytoplankton. Carbonate compensation depth, of course, only refers to the former; more on silica later. Because of our constant burning of our fossil fuels following the industrial revolution, we have dramatically increased the amount of \(\ce{CO2}\) in our atmosphere and essentially tilted the carbon cycle. In revising his resume, iwritegigs highlighted his soft skills such as his communication skills, ability to negotiate, patience and tactfulness. Calcium carbonate is the main chemical in the mineral calcite. 5- Calcium carbonate begins to precipitate into a. solid. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. WebCalcite compensation depth (CCD) The depth in the sea at which the rate of dissolution of solid calcium carbonate equals the rate of supply. Below the calcium carbonate compensation depth ( CCD ) calcareous ooze is completely dissolved. WebBelow the saturation, waters are undersaturated because of increasing solubility with depth and the release of CO 2 from organic matter decay and CaCO 3 will dissolve. 
 InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, Except where otherwise noted, data are given for materials in their. d. Siliceous ooze deposition. You can find mineral particles from land and outer space, particles from hydrothermal "black smokers" and the remains of microscopic living organisms, otherwise known as plankton. When CaCO3-shelledorganisms die, their skeletal remains begin sinking towards the bottom of the ocean.
InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, InChI=1/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2, Except where otherwise noted, data are given for materials in their. d. Siliceous ooze deposition. You can find mineral particles from land and outer space, particles from hydrothermal "black smokers" and the remains of microscopic living organisms, otherwise known as plankton. When CaCO3-shelledorganisms die, their skeletal remains begin sinking towards the bottom of the ocean.  A. Calcium carbonate begins to precipitate into a solid. At depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. https://www.britannica.com/science/calcite-compensation-depth. { "6.01:_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
A. Calcium carbonate begins to precipitate into a solid. At depths greater than 16,400 feet (5,000 metres), the calcium carbonate content decreases, and the calcareous deposits give way to red clay. https://www.britannica.com/science/calcite-compensation-depth. { "6.01:_Marine_Sediments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.