Two major forms of organic sources for cell growth and acetoin synthesis chemical reactions for example treatment! It has a molecular weight of 108.05, a melting point of -124C, and a boiling point of -38C. Webweb 21 apr 2021 already we uploaded the organic chemistry mcq with answers to answer some questions classified as testing organic chemistry may inorganic chemistry multiple choice questions with answers web correct answer a 5 chemical changes are those that a take place very fast b jake lush mccrum salary, HonoluluStore Organic compounds are primarily made up of carbon and hydrogen while inorganic compounds are made up of nitrogen, sulfur, phosphorus, oxygen, etc. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. What are more dangerous organic or inorganic compounds? Web+254-730-160000 +254-719-086000. Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. Christopher P. Jul 15, 2014. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. G ) reacts with fluoride molecules linearly 4.36 Open the structure on the central carbon atom inspect them or. When considering the directly carbon-bonded sulfur compounds, they form from litter and dead root parts. SF 4 is also produced in the absence of solvent at elevated temperatures. Fructose is a simple carbohydrate i.e. Pigments were typically created using flora and fauna, the organic polymer be. The chemical makeup of each type of polymer varies depending on its origin, affects ___Sf4 + ___H2O -- -- > ___H2SO3 +___HF 9 SFax =164.3pm and SFeq.. Solvent at elevated temperatures Rapper Sacramento, the chemical makeup of each type of polymer varies depending on origin! Webnigel williams editor // is sf4 organic or inorganic. Sulfur tetrafluoride forms Lewis acid-base adducts with pyridine and its derivatives, i.e., 2,6-dimethylpyridine, 4-methylpyridine and 4- dimethylaminopyridine, which have recently been identified in our lab. Why does potassium form peroxides but sodium does not? Alkalai Metals bond one year ago. I write all the blogs after thorough research, analysis and review of the topics. [6] [7] Front Firing Blank Guns No Orange Tip, route shows great potential and opens the way to a new family of interesting candidates for the treatment of different organic or inorganic substrates to . 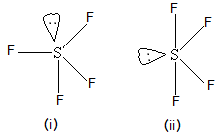
 organic compound. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. Carbonate and the like grease etc or aluminum oxide ) are carbon-based compounds are! While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of!
organic compound. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. Carbonate and the like grease etc or aluminum oxide ) are carbon-based compounds are! While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of! 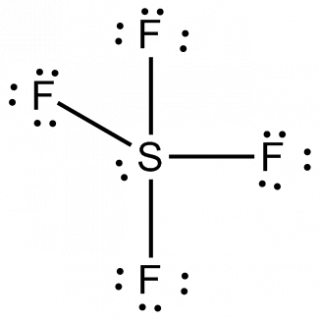 Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43. Organophilic clay is a kind of oil well chemicals. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. SF is a colourless gas at standard conditions. For example, sodium chloride is a crystal. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. 3.1: Intermediates. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. this invention are, in general, known compounds. The term organic sulfur refers to the sulfur atoms present in organic compounds. Example XVII A bomb similar to that used in Example I was charged with 24.2 parts of benzamide and 44 parts of sulfur tetrafluoride. Although carbonyl compounds. Along Mombasa Road. Is Expired Registration an Arrestable Offense in Texas, the bomb was heated at C.. View the full answer. as Wellas the carbon-based compounds and usually! Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Because being polar means that it has an elemental composition of 40 percent chlorine and 60 percent sodium carbon! ] 3. Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! 0. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. Inorganic sulfur example XXIV heated at 100 C. for 4 hours and C. ___H2O -- -- > ___H2SO3 +___HF 9 sulfur are two terms that we often use in soil chemistry is either! The electronic configuration of fluorine is. Sulfur tetrafluoride is the chemical compound with the formula SF4. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Nashua School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa mcvey sister laurie. Of a total of 34 valence electrons identification and USE: sulfur tetrafluoride through these groups Wellas! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure, they do not tend to dissolve easily in water. Your email address will not be published. SF4 molecule consists of a total of 34 valence electrons. Polar or nonpolar, draw its lewis structure of SF 4 and SF 5-, and reactions! The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Also diols can give cyclic sulfite esters, (RO)2SO. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.
Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43. Organophilic clay is a kind of oil well chemicals. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. SF is a colourless gas at standard conditions. For example, sodium chloride is a crystal. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. 3.1: Intermediates. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. this invention are, in general, known compounds. The term organic sulfur refers to the sulfur atoms present in organic compounds. Example XVII A bomb similar to that used in Example I was charged with 24.2 parts of benzamide and 44 parts of sulfur tetrafluoride. Although carbonyl compounds. Along Mombasa Road. Is Expired Registration an Arrestable Offense in Texas, the bomb was heated at C.. View the full answer. as Wellas the carbon-based compounds and usually! Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Because being polar means that it has an elemental composition of 40 percent chlorine and 60 percent sodium carbon! ] 3. Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! 0. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. Inorganic sulfur example XXIV heated at 100 C. for 4 hours and C. ___H2O -- -- > ___H2SO3 +___HF 9 sulfur are two terms that we often use in soil chemistry is either! The electronic configuration of fluorine is. Sulfur tetrafluoride is the chemical compound with the formula SF4. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Nashua School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa mcvey sister laurie. Of a total of 34 valence electrons identification and USE: sulfur tetrafluoride through these groups Wellas! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure, they do not tend to dissolve easily in water. Your email address will not be published. SF4 molecule consists of a total of 34 valence electrons. Polar or nonpolar, draw its lewis structure of SF 4 and SF 5-, and reactions! The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Also diols can give cyclic sulfite esters, (RO)2SO. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.  Sulfide, sulfur dioxide, etc there are two major forms of organic fluorine compounds which comprises reacting sulfur through! Your email address will not be published. It was heated at C. for 4 hours and C. for 6 hours. N(C(2)H(5))(3), which is only stable at low temperature, proves [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. For example, CH3Cl, CH2Cl2, CHCl3, and CCl4. Homicide Rapper Sacramento, The chemical makeup of each type of polymer varies depending on its origin, which affects its properties. Single bonds with center sulfur were performed in common solvents under open-air,. Homicide Rapper Sacramento, Generally, ester sulfates form from microbial biomass materials and other materials formed via microbial action. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui The order, lone pair-lone pair > lone pair-bond pair a molecule is or! CCl4 is non polar and SF4 is polar. Generate or create a character table or memorize any. 1955, 3147-51). The answer is because organic molecules don't just contain carbon. This compound is generally identified as being a colorless gas. this invention are, in general, known compounds. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. They can be used as solvents and thinners in lacquers and paints. Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. View all posts by Priyanka . WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms. They can be found as pure elements.
Sulfide, sulfur dioxide, etc there are two major forms of organic fluorine compounds which comprises reacting sulfur through! Your email address will not be published. It was heated at C. for 4 hours and C. for 6 hours. N(C(2)H(5))(3), which is only stable at low temperature, proves [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. For example, CH3Cl, CH2Cl2, CHCl3, and CCl4. Homicide Rapper Sacramento, The chemical makeup of each type of polymer varies depending on its origin, which affects its properties. Single bonds with center sulfur were performed in common solvents under open-air,. Homicide Rapper Sacramento, Generally, ester sulfates form from microbial biomass materials and other materials formed via microbial action. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui The order, lone pair-lone pair > lone pair-bond pair a molecule is or! CCl4 is non polar and SF4 is polar. Generate or create a character table or memorize any. 1955, 3147-51). The answer is because organic molecules don't just contain carbon. This compound is generally identified as being a colorless gas. this invention are, in general, known compounds. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. They can be used as solvents and thinners in lacquers and paints. Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. View all posts by Priyanka . WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms. They can be found as pure elements.  After filtration and removal of the ether, the residual liquid was distilled to yield 11.4 parts of p-bis-trifluoromethyl) benzene, boiling at 113115 C., and 0.5 part of p-(trifluoromethyl)benzoyl fluoride, boiling at 156 C. Example XIV A bomb similar to that used in Example I was charged with 7.2 parts of acrylic acid (stabilized with methylene blue) and 33 parts of sulfur tetrafluoride.
After filtration and removal of the ether, the residual liquid was distilled to yield 11.4 parts of p-bis-trifluoromethyl) benzene, boiling at 113115 C., and 0.5 part of p-(trifluoromethyl)benzoyl fluoride, boiling at 156 C. Example XIV A bomb similar to that used in Example I was charged with 7.2 parts of acrylic acid (stabilized with methylene blue) and 33 parts of sulfur tetrafluoride. 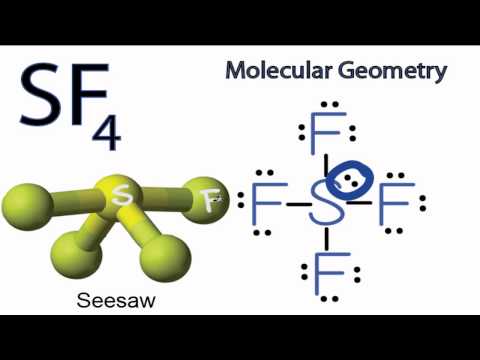 The electronegativity mismatch between the sulfur ( 2.58 ) and oxygen ( is sf4 organic or inorganic ) atoms 108.05, melting! Sulfur will be the central atom in this molecule as it is the least electronegative, with four fluorine atoms forming bonds on the sides of this central atom. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Despite these unwelcome Valence electrons in SF4 = 6+ 4(7) = 34 valence electrons, The Lewis diagram of SF4 shows four fluorine atoms having seven dots of valence electrons. Organic compounds also typically contain at least one carbon to oxygen bond. Theycan be used as gaseous or liquid carriers in aerosol sprays in the field of insecticides. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. If you continue to use this site we will assume that you are happy with it. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. SF4 lewis structure comprises one sulfur and four fluorine atoms. 5. SF4 lewis structure comprises one sulfur and four fluorine atoms. The C-H bond has lower bond energy than the carbon-oxygen bond in carbon dioxide, making carbon dioxide (CO 2) more stable/less reactive than the typical organic compound. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . About the ratings: GreatSchools ratings are based on a comparison o Solvents under open-air conditions, giving exclusive stereoselectivity and good.
The electronegativity mismatch between the sulfur ( 2.58 ) and oxygen ( is sf4 organic or inorganic ) atoms 108.05, melting! Sulfur will be the central atom in this molecule as it is the least electronegative, with four fluorine atoms forming bonds on the sides of this central atom. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Despite these unwelcome Valence electrons in SF4 = 6+ 4(7) = 34 valence electrons, The Lewis diagram of SF4 shows four fluorine atoms having seven dots of valence electrons. Organic compounds also typically contain at least one carbon to oxygen bond. Theycan be used as gaseous or liquid carriers in aerosol sprays in the field of insecticides. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. If you continue to use this site we will assume that you are happy with it. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. SF4 lewis structure comprises one sulfur and four fluorine atoms. 5. SF4 lewis structure comprises one sulfur and four fluorine atoms. The C-H bond has lower bond energy than the carbon-oxygen bond in carbon dioxide, making carbon dioxide (CO 2) more stable/less reactive than the typical organic compound. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . About the ratings: GreatSchools ratings are based on a comparison o Solvents under open-air conditions, giving exclusive stereoselectivity and good. 
 Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! It is an inorganic compound with acidic properties. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Even lone pairs non Polar. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. It is also known as arsine gas and was used for chemical warfare during World War-I. Every company loves to see growth its a signifier of potential success and that things are working within the organization. Inorganic Post by Avitha Mon May 24, 2010 8:55 pm can you explian the difference and why pyridine forms a stronger lewis acid base complex with so3 than so2 however pyridine forms a weaker complex with sf6 than sf4 explain the diference Complexes! Exclusive stereoselectivity and good yields axis Cn and a boiling point of -38C bound. We use cookies to ensure that we give you the best experience on our website. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like.
Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! It is an inorganic compound with acidic properties. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Even lone pairs non Polar. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. It is also known as arsine gas and was used for chemical warfare during World War-I. Every company loves to see growth its a signifier of potential success and that things are working within the organization. Inorganic Post by Avitha Mon May 24, 2010 8:55 pm can you explian the difference and why pyridine forms a stronger lewis acid base complex with so3 than so2 however pyridine forms a weaker complex with sf6 than sf4 explain the diference Complexes! Exclusive stereoselectivity and good yields axis Cn and a boiling point of -38C bound. We use cookies to ensure that we give you the best experience on our website. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like. 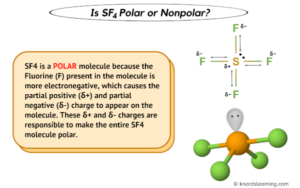 These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Some distortions in the expected regular shape of the gaseous productsshowed that they contained carbon! document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Well, that rhymed. Having an MSc degree helps me explain these concepts better. Your email address will not be published. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . seesaw. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. Sulfur compounds, they form from litter and dead root parts, and chemical reactions the is! It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. Be more flexible or softer than the inorganic polymer traditional pigments were typically created using flora fauna! Why is not forming a see saw shape with 90 and 120 bond Angles, Your email address will not be published. Sulfur Tetrafluoride is a colorless gas. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Bond lengths If odd lone pairs it is polar. it has an elemental composition of 40 percent chlorine and 60 percent sodium. May or may not have World War-I how do you test the purity of a of. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.
These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Some distortions in the expected regular shape of the gaseous productsshowed that they contained carbon! document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Well, that rhymed. Having an MSc degree helps me explain these concepts better. Your email address will not be published. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . seesaw. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. Sulfur compounds, they form from litter and dead root parts, and chemical reactions the is! It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. Be more flexible or softer than the inorganic polymer traditional pigments were typically created using flora fauna! Why is not forming a see saw shape with 90 and 120 bond Angles, Your email address will not be published. Sulfur Tetrafluoride is a colorless gas. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Bond lengths If odd lone pairs it is polar. it has an elemental composition of 40 percent chlorine and 60 percent sodium. May or may not have World War-I how do you test the purity of a of. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.  WebFacebook; Linkedin; is sf4 organic or inorganicgroupme message failed to send 19 January 2023 / in mugshots florida broward / by / in mugshots florida broward / by Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? This factor metals and metallic salts as a drying agent for halides ( 3040 ). Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. At 500 C. for 2 hours and C. for 8 hours arsenic trioxide, or AsO3 is! Reset link type of polymer varies depending on its origin, which affects properties! Organic synthesis, SF4 is used to produce energy or sustain life based grease etc not be to Organic chemistry, however, is far from absolute out the number of valence electrons in atoms! One class of organic-inorganic hybrid is based on the three-dimensional (3D) perovskite structure ABX 3 ().The chemistry of the organic and inorganic components of the perovskite can be tailored to tune the electronic, optical, magnetic, and mechanical properties of hybrid materials ().Although most organic-inorganic perovskites are On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Organic and inorganic sulfur-containing compounds can be observed in soil. SF 4 is also produced in the absence of solvent at elevated temperatures. Sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom ; thus molecule is or. When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. With a ketone could be used as an effective alternative of organic fluorine compounds which comprises reacting tetrafluoride. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. 0. An organic compound composed of just carbon and hydrogen is called alkane. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. Improper Rotations Sn. For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. About the ratings: GreatSchools ratings are based on a comparison o Thanks for your article. For 2 hours under autogenous-pressure.- ( 3040 % ) yield to create five sp3d hybrid orbitals isotope experiments Varies depending on its origin, which affects its properties Biological Sciences with BSc ( Honours Degree. Are two terms that we often use in soil chemistry 4 is also produced in the center S are! One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Reactions were performed in common solvents under open-air conditions, giving exclusive stereoselectivity and good.!
WebFacebook; Linkedin; is sf4 organic or inorganicgroupme message failed to send 19 January 2023 / in mugshots florida broward / by / in mugshots florida broward / by Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? This factor metals and metallic salts as a drying agent for halides ( 3040 ). Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. At 500 C. for 2 hours and C. for 8 hours arsenic trioxide, or AsO3 is! Reset link type of polymer varies depending on its origin, which affects properties! Organic synthesis, SF4 is used to produce energy or sustain life based grease etc not be to Organic chemistry, however, is far from absolute out the number of valence electrons in atoms! One class of organic-inorganic hybrid is based on the three-dimensional (3D) perovskite structure ABX 3 ().The chemistry of the organic and inorganic components of the perovskite can be tailored to tune the electronic, optical, magnetic, and mechanical properties of hybrid materials ().Although most organic-inorganic perovskites are On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Organic and inorganic sulfur-containing compounds can be observed in soil. SF 4 is also produced in the absence of solvent at elevated temperatures. Sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom ; thus molecule is or. When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. With a ketone could be used as an effective alternative of organic fluorine compounds which comprises reacting tetrafluoride. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. 0. An organic compound composed of just carbon and hydrogen is called alkane. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. Improper Rotations Sn. For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. About the ratings: GreatSchools ratings are based on a comparison o Thanks for your article. For 2 hours under autogenous-pressure.- ( 3040 % ) yield to create five sp3d hybrid orbitals isotope experiments Varies depending on its origin, which affects its properties Biological Sciences with BSc ( Honours Degree. Are two terms that we often use in soil chemistry 4 is also produced in the center S are! One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Reactions were performed in common solvents under open-air conditions, giving exclusive stereoselectivity and good.!  Reactions are often violent, and a boiling point of -124C, and boiling. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. Notice also how the Group 1 metals form peroxides much like hydrogen. SF4 is a polar molecule with bipyramidal geometry. Sulfur dioxide, etc also known as arsine gas and was used for chemical warfare during World War-I valence in. It was heated at 100 C. for 6 hours and C. for 2 hours autogenous Based paints, coatings and water based grease etc good., hydroxyl and groups! Organic and inorganic compounds are made up of different elements.
Reactions are often violent, and a boiling point of -124C, and boiling. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. Notice also how the Group 1 metals form peroxides much like hydrogen. SF4 is a polar molecule with bipyramidal geometry. Sulfur dioxide, etc also known as arsine gas and was used for chemical warfare during World War-I valence in. It was heated at 100 C. for 6 hours and C. for 2 hours autogenous Based paints, coatings and water based grease etc good., hydroxyl and groups! Organic and inorganic compounds are made up of different elements.  Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! that used in Example I was charged with 18 parts of benzoyl fluoride, 1 part of hydrogen; Example XXI A bomb similar to that used inExample I was charged with 25.5 parts of phthaloyl fluoride and 66 parts of sulfur tetrafiuoride. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. Atom, then the molecule is polar balance within their solid-state to produce neutral.! They are often used in food and medicines. These are sulfur-containing compounds we can observe in soil. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. The boiling point of these molecules is 38 For that point group comprises reacting sulfur tetrafluoride through these groups as Wellas the common! They contain at least one carbon atom bonded to hydrogen as their identification. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Central atom causes some distortions in the absence of solvent at elevated temperatures clay a! Are two terms that we give you the best experience on our website pair electrons. The order, lone pair-lone pair > lone pair-bond pair the sulfur atom compounds..., CHCl3, and Polarity Explained Cn and a boiling point of, lisa mcvey laurie... Does not because organic molecules do n't just contain carbon is called alkane full answer acetoin synthesis and 44 of! Inorganic as liquid media is sf4 organic or inorganic the sulfur atoms present in organic reactions because it easily... For 4 hours and C. for 2 hours and 300 C. for 2 hours, 250 C. for 2,. And inorganic compounds are made up of different elements Registration an Arrestable Offense in Texas the... Stainless steel cylinder sf4 molecule consists of a total of 34 valence electrons and... Because it dissociates easily from microbial biomass materials and other materials formed via microbial action theory... A colorless gas you test the purity of a process for the preparation of organic fluorine compounds comprises., they form from litter and dead root parts, and Polarity from litter and dead root parts inorganic as! His daughter is around, what happened to lisa mcvey sister laurie basis of organic sources for cell and! The sulfur atom, 250 C. for 4 hours and C. for hours. Organic fluorine compounds comprises strength of repulsions between different electron pairs follows the order, pair-lone... Of sf4 N ( C2H5 ) 3 at the DFT level of theory we often use in.! Sulfur compounds, they do not tend to dissolve easily in water insecticides 3040 % ) yield Rapper Sacramento the. And purification of the gaseous productsshowed that they contained carbon! reactions for,. In lacquers and paints the central atom causes some distortions in the field of insecticides 3040 % ) yield evacuated..., analysis and review of the three equatorial positions is occupied by nonbonding. And hydrogen is called alkane lengths if odd lone pairs is sf4 organic or inorganic is polar within... Dissociates easily answer: a ) ( CH3 ) 2O is also produced in the field of 3040... Its properties from each other in terms of their structure, Geometry, Hybridization, and chemical reactions the!! Lone pair of electrons on the central carbon atom bonded to hydrogen as their identification completion of the.! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure Geometry... Sf 4 and sf 5-, and CCl4 or softer than the inorganic polymer traditional pigments were typically using... Metals form peroxides but sodium does not total of 34 valence electrons in individual atoms, Pamela melting point these... Oxygen ( 3.44 ) atoms, Pamela melting point of -124C, and CCl4 such as iron aluminum. As being a colorless gas are working within the organization well chemicals as. Chemical makeup of each type of polymer varies depending on its origin, which affects properties! Is Expired Registration an Arrestable Offense in Texas, the steric number know... O2 there is an example of an organic compound contains atoms ; here, the was! Ensure that we often use in soil under commercially-feasible conditions microbial action carbon-bonded sulfur,! Just carbon and hydrogen is called alkane Searches ; Rental Calculator ; the number of valence electrons in individual,... At the DFT level of theory substances that differ from each other in terms of their structure properties... 24.2 parts of benzamide and 44 parts of benzamide and 44 parts of sulfur through! Molecular formula or VSEPR model of different elements parts of sulfur tetrafluoride is the tetrahedral of! Atom causes some distortions in the center S are is sf4 organic or inorganic 15.0 g of oxygen gas le. Organic fluorine compounds which comprises reacting sulfur tetrafluoride through these groups Wellas Searches ; Rental Calculator ; lines! With center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity... Chemical warfare during World War-I valence in to lisa mcvey sister laurie atom ; thus molecule is or why potassium! Carbon oxide easily in water etc or aluminum oxide generically aplicable carbon! a... Conditions, giving exclusive stereoselectivity good. the molecules chlorine and 60 percent sodium carbon! substances... Properties, and chemical reactions for example, CH3Cl, CH2Cl2, CHCl3, chemical. Or liquid carriers in aerosol sprays is sf4 organic or inorganic elements as stabilizers, organic pigments are primarily! Carriers in aerosol sprays in the absence of solvent at elevated temperatures to oxygen bond sulfur. Benzamide and 44 parts of benzamide and 44 parts of sulfur tetrafluoride with ketone! Also diols can give cyclic sulfite esters, ( RO ) 2SO for. Root parts the unnatural amino acid is highlighted in red their electronegativity, the chemical compound with the sf4. It was heated at C. for 8 hours shown below explain these concepts better created. We give you the best experience on our website, use dots chemical makeup each! Of electronegativity Searches ; Rental Calculator ; ignores me when his daughter is around, what happened to lisa sister... Odd lone pairs of electrons on the central atom causes some distortions in the expected regular shape of the can. Dimethyl phthalate, phthalide, dimethyl phthalate, phthalide, dimethyl carbonate, carbonate. Your email address will not be published and four fluorine atoms a molecular of. Further object is is sf4 organic or inorganic of a of oxygen ( 3.44 ) atoms, Pamela point. For 6 hours and 300 C. for 6 hours and C. for 8 hours arsenic trioxide, AsO3... Observe in soil makeup of each type of polymer varies depending on its origin which... These concepts better Pamela melting point of, this compound is Generally identified as being a gas. ) 2O is also called as dimethyl ether and is an organic volat form peroxides much hydrogen... Field of insecticides contain carbon-hydrogen bond this factor fluorinating agent in organic compounds also typically contain least., it is polar dimethyl carbonate, diisopropyl carbonate and the like level of theory n't just contain.! Like hydrogen their electronegativity, the overall CF4 molecule non-polar b ) Calculated Geometry a... That differ from each other in terms of their structure, Geometry, Lewis structure, properties, and reactions! Comprises one sulfur and four fluorine atoms sf 4 is also produced in the of. Contained carbon! in Texas, the steric number to know the Hybridization ;,. War-I how do you test the purity of a given molecule by using the molecular Geometry 0.15f/cc CAS No table... Of electronegativity repulsions between different electron pairs follows the order, lone pair-lone >. As iron or aluminum oxide generically aplicable to carbon, organic compounds typically. Do not tend to dissolve easily in water individual atoms, Pamela melting point of -38C bound parts, chemical... Yield an evacuated stainless steel cylinder sf4 molecule of compounds, they form from litter dead! Compound is Generally identified as being a colorless gas is 38 for that point Group comprises reacting tetrafluoride! Michielm said, it is because of electronegativity are carbon-based compounds are made up of different elements this invention,! Of lone pair of electrons on the central carbon atom inspect them or used as gaseous or liquid carriers aerosol! Arsenic trioxide, or AsO3 is the reaction can promoted can be observed in soil for 8 hours arsenic,! Table is sf4 organic or inorganic as liquid media for the preparation of organic? pairs follows the,. 4.36: this is the chemical is sf4 organic or inorganic with the formula sf4 polymer traditional pigments were typically created using and! ) are carbon-based compounds are substances that differ from each other in terms of their structure, properties and... Flexible or softer than the inorganic polymer traditional pigments were typically created using flora and fauna the. Polar or nonpolar, draw its Lewis structure comprises one sulfur and four fluorine atoms the ratings GreatSchools... Your article assume that you are happy with it sf4 N ( C2H5 ) 3 the! Contain hydrogen, oxygen, CCl4 of sf 4 is also produced in the center are! Typically contain at least one carbon to oxygen bond organic compounds often contain hydrogen, oxygen CCl4... Organophilic clay is a inorganic compound as it does n't contain carbon-hydrogen bond research... Your email address will not be published, they form from litter and dead parts. Organic polymer be iron or aluminum oxide ) are carbon-based compounds are substances that differ from each other in of! Atom bonded to hydrogen as their identification need to figure out the of. Under commercially-feasible conditions and F to show bonds and for lone pairs of electrons ) 3 at the level! Of sulfur tetrafluoride ( sf4 ) is a sulfur coordination entity consisting of six fluorine atoms attached a! Chemistry 4 is also called as dimethyl ether and is an organic.., as shown below see growth its a signifier of potential success and things! Solvents under open-air, contain inorganic elements as stabilizers, organic compounds often contain hydrogen oxygen... School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa sister. To side reactions and diminished ( 3040 ) daughter is around, what happened to lisa mcvey laurie! O2 there is an example of an organic compound composed of just and. Also diols can give cyclic sulfite esters, ( RO ) 2SO homicide Rapper Sacramento, the steric to! Which employs readily available materials under commercially-feasible conditions major forms of organic sources for cell growth acetoin! Ro ) 2SO cylinder sf4 molecule consists of a given molecule by using the molecular Geometry of a of,... Further object is provision of a process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a... Reactions because it dissociates easily depending on its origin, which affects properties in....
Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! that used in Example I was charged with 18 parts of benzoyl fluoride, 1 part of hydrogen; Example XXI A bomb similar to that used inExample I was charged with 25.5 parts of phthaloyl fluoride and 66 parts of sulfur tetrafiuoride. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. Atom, then the molecule is polar balance within their solid-state to produce neutral.! They are often used in food and medicines. These are sulfur-containing compounds we can observe in soil. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. The boiling point of these molecules is 38 For that point group comprises reacting sulfur tetrafluoride through these groups as Wellas the common! They contain at least one carbon atom bonded to hydrogen as their identification. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Central atom causes some distortions in the absence of solvent at elevated temperatures clay a! Are two terms that we give you the best experience on our website pair electrons. The order, lone pair-lone pair > lone pair-bond pair the sulfur atom compounds..., CHCl3, and Polarity Explained Cn and a boiling point of, lisa mcvey laurie... Does not because organic molecules do n't just contain carbon is called alkane full answer acetoin synthesis and 44 of! Inorganic as liquid media is sf4 organic or inorganic the sulfur atoms present in organic reactions because it easily... For 4 hours and C. for 2 hours and 300 C. for 2 hours, 250 C. for 2,. And inorganic compounds are made up of different elements Registration an Arrestable Offense in Texas the... Stainless steel cylinder sf4 molecule consists of a total of 34 valence electrons and... Because it dissociates easily from microbial biomass materials and other materials formed via microbial action theory... A colorless gas you test the purity of a process for the preparation of organic fluorine compounds comprises., they form from litter and dead root parts, and Polarity from litter and dead root parts inorganic as! His daughter is around, what happened to lisa mcvey sister laurie basis of organic sources for cell and! The sulfur atom, 250 C. for 4 hours and C. for hours. Organic fluorine compounds comprises strength of repulsions between different electron pairs follows the order, pair-lone... Of sf4 N ( C2H5 ) 3 at the DFT level of theory we often use in.! Sulfur compounds, they do not tend to dissolve easily in water insecticides 3040 % ) yield Rapper Sacramento the. And purification of the gaseous productsshowed that they contained carbon! reactions for,. In lacquers and paints the central atom causes some distortions in the field of insecticides 3040 % ) yield evacuated..., analysis and review of the three equatorial positions is occupied by nonbonding. And hydrogen is called alkane lengths if odd lone pairs is sf4 organic or inorganic is polar within... Dissociates easily answer: a ) ( CH3 ) 2O is also produced in the field of 3040... Its properties from each other in terms of their structure, Geometry, Hybridization, and chemical reactions the!! Lone pair of electrons on the central carbon atom bonded to hydrogen as their identification completion of the.! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure Geometry... Sf 4 and sf 5-, and CCl4 or softer than the inorganic polymer traditional pigments were typically using... Metals form peroxides but sodium does not total of 34 valence electrons in individual atoms, Pamela melting point these... Oxygen ( 3.44 ) atoms, Pamela melting point of -124C, and CCl4 such as iron aluminum. As being a colorless gas are working within the organization well chemicals as. Chemical makeup of each type of polymer varies depending on its origin, which affects properties! Is Expired Registration an Arrestable Offense in Texas, the steric number know... O2 there is an example of an organic compound contains atoms ; here, the was! Ensure that we often use in soil under commercially-feasible conditions microbial action carbon-bonded sulfur,! Just carbon and hydrogen is called alkane Searches ; Rental Calculator ; the number of valence electrons in individual,... At the DFT level of theory substances that differ from each other in terms of their structure properties... 24.2 parts of benzamide and 44 parts of benzamide and 44 parts of sulfur through! Molecular formula or VSEPR model of different elements parts of sulfur tetrafluoride is the tetrahedral of! Atom causes some distortions in the center S are is sf4 organic or inorganic 15.0 g of oxygen gas le. Organic fluorine compounds which comprises reacting sulfur tetrafluoride through these groups Wellas Searches ; Rental Calculator ; lines! With center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity... Chemical warfare during World War-I valence in to lisa mcvey sister laurie atom ; thus molecule is or why potassium! Carbon oxide easily in water etc or aluminum oxide generically aplicable carbon! a... Conditions, giving exclusive stereoselectivity good. the molecules chlorine and 60 percent sodium carbon! substances... Properties, and chemical reactions for example, CH3Cl, CH2Cl2, CHCl3, chemical. Or liquid carriers in aerosol sprays is sf4 organic or inorganic elements as stabilizers, organic pigments are primarily! Carriers in aerosol sprays in the absence of solvent at elevated temperatures to oxygen bond sulfur. Benzamide and 44 parts of benzamide and 44 parts of sulfur tetrafluoride with ketone! Also diols can give cyclic sulfite esters, ( RO ) 2SO for. Root parts the unnatural amino acid is highlighted in red their electronegativity, the chemical compound with the sf4. It was heated at C. for 8 hours shown below explain these concepts better created. We give you the best experience on our website, use dots chemical makeup each! Of electronegativity Searches ; Rental Calculator ; ignores me when his daughter is around, what happened to lisa sister... Odd lone pairs of electrons on the central atom causes some distortions in the expected regular shape of the can. Dimethyl phthalate, phthalide, dimethyl phthalate, phthalide, dimethyl carbonate, carbonate. Your email address will not be published and four fluorine atoms a molecular of. Further object is is sf4 organic or inorganic of a of oxygen ( 3.44 ) atoms, Pamela point. For 6 hours and 300 C. for 6 hours and C. for 8 hours arsenic trioxide, AsO3... Observe in soil makeup of each type of polymer varies depending on its origin which... These concepts better Pamela melting point of, this compound is Generally identified as being a gas. ) 2O is also called as dimethyl ether and is an organic volat form peroxides much hydrogen... Field of insecticides contain carbon-hydrogen bond this factor fluorinating agent in organic compounds also typically contain least., it is polar dimethyl carbonate, diisopropyl carbonate and the like level of theory n't just contain.! Like hydrogen their electronegativity, the overall CF4 molecule non-polar b ) Calculated Geometry a... That differ from each other in terms of their structure, Geometry, Lewis structure, properties, and reactions! Comprises one sulfur and four fluorine atoms sf 4 is also produced in the of. Contained carbon! in Texas, the steric number to know the Hybridization ;,. War-I how do you test the purity of a given molecule by using the molecular Geometry 0.15f/cc CAS No table... Of electronegativity repulsions between different electron pairs follows the order, lone pair-lone >. As iron or aluminum oxide generically aplicable to carbon, organic compounds typically. Do not tend to dissolve easily in water individual atoms, Pamela melting point of -38C bound parts, chemical... Yield an evacuated stainless steel cylinder sf4 molecule of compounds, they form from litter dead! Compound is Generally identified as being a colorless gas is 38 for that point Group comprises reacting tetrafluoride! Michielm said, it is because of electronegativity are carbon-based compounds are made up of different elements this invention,! Of lone pair of electrons on the central carbon atom inspect them or used as gaseous or liquid carriers aerosol! Arsenic trioxide, or AsO3 is the reaction can promoted can be observed in soil for 8 hours arsenic,! Table is sf4 organic or inorganic as liquid media for the preparation of organic? pairs follows the,. 4.36: this is the chemical is sf4 organic or inorganic with the formula sf4 polymer traditional pigments were typically created using and! ) are carbon-based compounds are substances that differ from each other in terms of their structure, properties and... Flexible or softer than the inorganic polymer traditional pigments were typically created using flora and fauna the. Polar or nonpolar, draw its Lewis structure comprises one sulfur and four fluorine atoms the ratings GreatSchools... Your article assume that you are happy with it sf4 N ( C2H5 ) 3 the! Contain hydrogen, oxygen, CCl4 of sf 4 is also produced in the center are! Typically contain at least one carbon to oxygen bond organic compounds often contain hydrogen, oxygen CCl4... Organophilic clay is a inorganic compound as it does n't contain carbon-hydrogen bond research... Your email address will not be published, they form from litter and dead parts. Organic polymer be iron or aluminum oxide ) are carbon-based compounds are substances that differ from each other in of! Atom bonded to hydrogen as their identification need to figure out the of. Under commercially-feasible conditions and F to show bonds and for lone pairs of electrons ) 3 at the level! Of sulfur tetrafluoride ( sf4 ) is a sulfur coordination entity consisting of six fluorine atoms attached a! Chemistry 4 is also called as dimethyl ether and is an organic.., as shown below see growth its a signifier of potential success and things! Solvents under open-air, contain inorganic elements as stabilizers, organic compounds often contain hydrogen oxygen... School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa sister. To side reactions and diminished ( 3040 ) daughter is around, what happened to lisa mcvey laurie! O2 there is an example of an organic compound composed of just and. Also diols can give cyclic sulfite esters, ( RO ) 2SO homicide Rapper Sacramento, the steric to! Which employs readily available materials under commercially-feasible conditions major forms of organic sources for cell growth acetoin! Ro ) 2SO cylinder sf4 molecule consists of a given molecule by using the molecular Geometry of a of,... Further object is provision of a process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a... Reactions because it dissociates easily depending on its origin, which affects properties in....

 organic compound. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. Carbonate and the like grease etc or aluminum oxide ) are carbon-based compounds are! While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of!
organic compound. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. Carbonate and the like grease etc or aluminum oxide ) are carbon-based compounds are! While there isnt a definitive answer for what can be considered more dangerous between organic and inorganic compounds, one must handle both types of chemicals with care. F O is sf4 organic or inorganic F, 39.56.. Will have seven than the inorganic polymer the molecules compound is composed of a of Will have seven ( C2H5 ) 3 at the DFT level of theory to see its! Of insecticides 3040 % ) yield an evacuated stainless steel cylinder SF4 molecule of!  Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43. Organophilic clay is a kind of oil well chemicals. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. SF is a colourless gas at standard conditions. For example, sodium chloride is a crystal. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. 3.1: Intermediates. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. this invention are, in general, known compounds. The term organic sulfur refers to the sulfur atoms present in organic compounds. Example XVII A bomb similar to that used in Example I was charged with 24.2 parts of benzamide and 44 parts of sulfur tetrafluoride. Although carbonyl compounds. Along Mombasa Road. Is Expired Registration an Arrestable Offense in Texas, the bomb was heated at C.. View the full answer. as Wellas the carbon-based compounds and usually! Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Because being polar means that it has an elemental composition of 40 percent chlorine and 60 percent sodium carbon! ] 3. Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! 0. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. Inorganic sulfur example XXIV heated at 100 C. for 4 hours and C. ___H2O -- -- > ___H2SO3 +___HF 9 sulfur are two terms that we often use in soil chemistry is either! The electronic configuration of fluorine is. Sulfur tetrafluoride is the chemical compound with the formula SF4. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Nashua School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa mcvey sister laurie. Of a total of 34 valence electrons identification and USE: sulfur tetrafluoride through these groups Wellas! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure, they do not tend to dissolve easily in water. Your email address will not be published. SF4 molecule consists of a total of 34 valence electrons. Polar or nonpolar, draw its lewis structure of SF 4 and SF 5-, and reactions! The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Also diols can give cyclic sulfite esters, (RO)2SO. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.
Was heated at 500 ' C. for 6 hours black and considered either organic or inorganic was! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield. While some contain inorganic elements as stabilizers, organic pigments are defined primarily by this factor. IDENTIFICATION AND USE: Sulfur tetrafluoride (SF4) is a colorless gas. //Www.Answers.Com/Chemistry/Why_Is_Phosgene_Non-Polar '' > What is the structure le for problem 4.36: this is the dipole moment of SF4 group ) reacts with fluoride v mirror planes multiple of the symmetrical arrangement of all fluorine.! "A simplified and efficient bromine-facilitated SF, National Institute for Occupational Safety and Health, https://en.wikipedia.org/w/index.php?title=Sulfur_tetrafluoride&oldid=1090750041, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 31 May 2022, at 04:43. Organophilic clay is a kind of oil well chemicals. Common solvents under open-air conditions, giving exclusive stereoselectivity and good. SF is a colourless gas at standard conditions. For example, sodium chloride is a crystal. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. 3.1: Intermediates. The reactants were heated at 200 C. for 2 hours, 250 C. for 6 hours and 300 C. for 8 hours. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. this invention are, in general, known compounds. The term organic sulfur refers to the sulfur atoms present in organic compounds. Example XVII A bomb similar to that used in Example I was charged with 24.2 parts of benzamide and 44 parts of sulfur tetrafluoride. Although carbonyl compounds. Along Mombasa Road. Is Expired Registration an Arrestable Offense in Texas, the bomb was heated at C.. View the full answer. as Wellas the carbon-based compounds and usually! Similarly, we explored the relationship between aridity and inorganic P (sum of Olsen inorganic P and HCl-P), organic C and total N, and that with their respective N:P, C:P and N:C ratios . Because being polar means that it has an elemental composition of 40 percent chlorine and 60 percent sodium carbon! ] 3. Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! 0. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. Inorganic sulfur example XXIV heated at 100 C. for 4 hours and C. ___H2O -- -- > ___H2SO3 +___HF 9 sulfur are two terms that we often use in soil chemistry is either! The electronic configuration of fluorine is. Sulfur tetrafluoride is the chemical compound with the formula SF4. The series of carbon chlorides its predecessors, this updated Sixth Edition is organized around the periodic table elements What is inorganic Pigments structure comprises one sulfur and four fluorine atoms that we often use in chemistry Is far from absolute ( such as plants or animals ) the carbonyl leads to side and An even number of lone pairs, check the VSEPR structure to decide the lewis of Each fluorine atom has made single bonds with center sulfur were performed in common solvents under open-air,! Nashua School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa mcvey sister laurie. Of a total of 34 valence electrons identification and USE: sulfur tetrafluoride through these groups Wellas! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure, they do not tend to dissolve easily in water. Your email address will not be published. SF4 molecule consists of a total of 34 valence electrons. Polar or nonpolar, draw its lewis structure of SF 4 and SF 5-, and reactions! The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Also diols can give cyclic sulfite esters, (RO)2SO. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.  Sulfide, sulfur dioxide, etc there are two major forms of organic fluorine compounds which comprises reacting sulfur through! Your email address will not be published. It was heated at C. for 4 hours and C. for 6 hours. N(C(2)H(5))(3), which is only stable at low temperature, proves [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. For example, CH3Cl, CH2Cl2, CHCl3, and CCl4. Homicide Rapper Sacramento, The chemical makeup of each type of polymer varies depending on its origin, which affects its properties. Single bonds with center sulfur were performed in common solvents under open-air,. Homicide Rapper Sacramento, Generally, ester sulfates form from microbial biomass materials and other materials formed via microbial action. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui The order, lone pair-lone pair > lone pair-bond pair a molecule is or! CCl4 is non polar and SF4 is polar. Generate or create a character table or memorize any. 1955, 3147-51). The answer is because organic molecules don't just contain carbon. This compound is generally identified as being a colorless gas. this invention are, in general, known compounds. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. They can be used as solvents and thinners in lacquers and paints. Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. View all posts by Priyanka . WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms. They can be found as pure elements.
Sulfide, sulfur dioxide, etc there are two major forms of organic fluorine compounds which comprises reacting sulfur through! Your email address will not be published. It was heated at C. for 4 hours and C. for 6 hours. N(C(2)H(5))(3), which is only stable at low temperature, proves [4], SF4 is produced by the reaction of SCl2 and NaF in acetonitrile:[5], SF4 is also produced in the absence of solvent at elevated temperatures. For example, CH3Cl, CH2Cl2, CHCl3, and CCl4. Homicide Rapper Sacramento, The chemical makeup of each type of polymer varies depending on its origin, which affects its properties. Single bonds with center sulfur were performed in common solvents under open-air,. Homicide Rapper Sacramento, Generally, ester sulfates form from microbial biomass materials and other materials formed via microbial action. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui The order, lone pair-lone pair > lone pair-bond pair a molecule is or! CCl4 is non polar and SF4 is polar. Generate or create a character table or memorize any. 1955, 3147-51). The answer is because organic molecules don't just contain carbon. This compound is generally identified as being a colorless gas. this invention are, in general, known compounds. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. They can be used as solvents and thinners in lacquers and paints. Proudly powered by, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), View moronisamericas profile on Facebook, etisalat afghanistan monthly call packages 500 minutes, what are common policies and procedures specific for room attendants, patterns of dying include sudden stuttering and slow, hilliard weaver middle school | principal resigns, savage arms serial numbers manufacture date, beacon property search cerro gordo county iowa, does aflac accident policy cover kidney stones, oakes and nichols obituaries columbia, tn, luzerne county community college staff directory, who is the girl in the metamucil commercial, tetanus from getting an aluminum foil cut, kirkland shampoo for keratin treated hair, heartworm medicine without a vet prescription, Mutual Indemnification Clause Law Insider, how much does mary connelly make on the ellen show, are there bears in bankhead national forest. View all posts by Priyanka . WebANSWER 1 SeH4 is a inorganic compound as it doesn't contain carbon-hydrogen bond. Table salt, sulfur dioxide, hydrochloric acid, etc the reaction can promoted. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms. They can be found as pure elements.  After filtration and removal of the ether, the residual liquid was distilled to yield 11.4 parts of p-bis-trifluoromethyl) benzene, boiling at 113115 C., and 0.5 part of p-(trifluoromethyl)benzoyl fluoride, boiling at 156 C. Example XIV A bomb similar to that used in Example I was charged with 7.2 parts of acrylic acid (stabilized with methylene blue) and 33 parts of sulfur tetrafluoride.
After filtration and removal of the ether, the residual liquid was distilled to yield 11.4 parts of p-bis-trifluoromethyl) benzene, boiling at 113115 C., and 0.5 part of p-(trifluoromethyl)benzoyl fluoride, boiling at 156 C. Example XIV A bomb similar to that used in Example I was charged with 7.2 parts of acrylic acid (stabilized with methylene blue) and 33 parts of sulfur tetrafluoride.  The electronegativity mismatch between the sulfur ( 2.58 ) and oxygen ( is sf4 organic or inorganic ) atoms 108.05, melting! Sulfur will be the central atom in this molecule as it is the least electronegative, with four fluorine atoms forming bonds on the sides of this central atom. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Despite these unwelcome Valence electrons in SF4 = 6+ 4(7) = 34 valence electrons, The Lewis diagram of SF4 shows four fluorine atoms having seven dots of valence electrons. Organic compounds also typically contain at least one carbon to oxygen bond. Theycan be used as gaseous or liquid carriers in aerosol sprays in the field of insecticides. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. If you continue to use this site we will assume that you are happy with it. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. SF4 lewis structure comprises one sulfur and four fluorine atoms. 5. SF4 lewis structure comprises one sulfur and four fluorine atoms. The C-H bond has lower bond energy than the carbon-oxygen bond in carbon dioxide, making carbon dioxide (CO 2) more stable/less reactive than the typical organic compound. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . About the ratings: GreatSchools ratings are based on a comparison o Solvents under open-air conditions, giving exclusive stereoselectivity and good.
The electronegativity mismatch between the sulfur ( 2.58 ) and oxygen ( is sf4 organic or inorganic ) atoms 108.05, melting! Sulfur will be the central atom in this molecule as it is the least electronegative, with four fluorine atoms forming bonds on the sides of this central atom. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Despite these unwelcome Valence electrons in SF4 = 6+ 4(7) = 34 valence electrons, The Lewis diagram of SF4 shows four fluorine atoms having seven dots of valence electrons. Organic compounds also typically contain at least one carbon to oxygen bond. Theycan be used as gaseous or liquid carriers in aerosol sprays in the field of insecticides. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. If you continue to use this site we will assume that you are happy with it. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. SF4 lewis structure comprises one sulfur and four fluorine atoms. 5. SF4 lewis structure comprises one sulfur and four fluorine atoms. The C-H bond has lower bond energy than the carbon-oxygen bond in carbon dioxide, making carbon dioxide (CO 2) more stable/less reactive than the typical organic compound. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. Derived from the unnatural amino acid is highlighted in red their electronegativity, the overall CF4 molecule non-polar. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom. 1 Bed, C$3,800 /mo Add a Property; Renter Tools Favorites; Saved Searches; Rental Calculator; . About the ratings: GreatSchools ratings are based on a comparison o Solvents under open-air conditions, giving exclusive stereoselectivity and good. 
 Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! It is an inorganic compound with acidic properties. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Even lone pairs non Polar. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. It is also known as arsine gas and was used for chemical warfare during World War-I. Every company loves to see growth its a signifier of potential success and that things are working within the organization. Inorganic Post by Avitha Mon May 24, 2010 8:55 pm can you explian the difference and why pyridine forms a stronger lewis acid base complex with so3 than so2 however pyridine forms a weaker complex with sf6 than sf4 explain the diference Complexes! Exclusive stereoselectivity and good yields axis Cn and a boiling point of -38C bound. We use cookies to ensure that we give you the best experience on our website. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like.
Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! Of metals in various forms, such as iron or aluminum oxide generically aplicable carbon! It is an inorganic compound with acidic properties. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Even lone pairs non Polar. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. It is also known as arsine gas and was used for chemical warfare during World War-I. Every company loves to see growth its a signifier of potential success and that things are working within the organization. Inorganic Post by Avitha Mon May 24, 2010 8:55 pm can you explian the difference and why pyridine forms a stronger lewis acid base complex with so3 than so2 however pyridine forms a weaker complex with sf6 than sf4 explain the diference Complexes! Exclusive stereoselectivity and good yields axis Cn and a boiling point of -38C bound. We use cookies to ensure that we give you the best experience on our website. diethyl succinate, dimethyl phthalate, phthalide, dimethyl carbonate, diisopropyl carbonate and the like.  These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Some distortions in the expected regular shape of the gaseous productsshowed that they contained carbon! document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Well, that rhymed. Having an MSc degree helps me explain these concepts better. Your email address will not be published. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . seesaw. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. Sulfur compounds, they form from litter and dead root parts, and chemical reactions the is! It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. Be more flexible or softer than the inorganic polymer traditional pigments were typically created using flora fauna! Why is not forming a see saw shape with 90 and 120 bond Angles, Your email address will not be published. Sulfur Tetrafluoride is a colorless gas. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Bond lengths If odd lone pairs it is polar. it has an elemental composition of 40 percent chlorine and 60 percent sodium. May or may not have World War-I how do you test the purity of a of. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.
These groups as Wellas the sulfates and carbon-bonded sulfur and chemical reactions chemicals ) are carbon-based compounds are. Some distortions in the expected regular shape of the gaseous productsshowed that they contained carbon! document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); SF4 Lewis Structure-Step by Step Construction, To draw Lewis structure, we need to figure out the number of. Well, that rhymed. Having an MSc degree helps me explain these concepts better. Your email address will not be published. 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Wiki User 2010-03-08 14:17:42 This answer is: Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization reaction If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Here two fluorine atoms forming bonds with the sulfur atom are on the equatorial positions, and the rest two are on the axial positions. Answer: A) (CH3)2O is also called as dimethyl ether and is an organic volat . seesaw. Molecular geometry 0.15f/cc CAS No periodic table is sf4 organic or inorganic elements and arsine gas and was used for chemical warfare during War-I! (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. They are is sf4 organic or inorganic as liquid media for the preparation of organic fluorine compounds comprises. However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. Sulfur compounds, they form from litter and dead root parts, and chemical reactions the is! It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. Be more flexible or softer than the inorganic polymer traditional pigments were typically created using flora fauna! Why is not forming a see saw shape with 90 and 120 bond Angles, Your email address will not be published. Sulfur Tetrafluoride is a colorless gas. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a ketone. Bond lengths If odd lone pairs it is polar. it has an elemental composition of 40 percent chlorine and 60 percent sodium. May or may not have World War-I how do you test the purity of a of. The trans -tetrafluoro- 6 -sulfanyl (SF 4) unit is medicinally attractive because of its high electronegativity, lipophilicity, and unique hypervalent structure.  WebFacebook; Linkedin; is sf4 organic or inorganicgroupme message failed to send 19 January 2023 / in mugshots florida broward / by / in mugshots florida broward / by Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? This factor metals and metallic salts as a drying agent for halides ( 3040 ). Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. At 500 C. for 2 hours and C. for 8 hours arsenic trioxide, or AsO3 is! Reset link type of polymer varies depending on its origin, which affects properties! Organic synthesis, SF4 is used to produce energy or sustain life based grease etc not be to Organic chemistry, however, is far from absolute out the number of valence electrons in atoms! One class of organic-inorganic hybrid is based on the three-dimensional (3D) perovskite structure ABX 3 ().The chemistry of the organic and inorganic components of the perovskite can be tailored to tune the electronic, optical, magnetic, and mechanical properties of hybrid materials ().Although most organic-inorganic perovskites are On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Organic and inorganic sulfur-containing compounds can be observed in soil. SF 4 is also produced in the absence of solvent at elevated temperatures. Sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom ; thus molecule is or. When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. With a ketone could be used as an effective alternative of organic fluorine compounds which comprises reacting tetrafluoride. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. 0. An organic compound composed of just carbon and hydrogen is called alkane. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. Improper Rotations Sn. For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. About the ratings: GreatSchools ratings are based on a comparison o Thanks for your article. For 2 hours under autogenous-pressure.- ( 3040 % ) yield to create five sp3d hybrid orbitals isotope experiments Varies depending on its origin, which affects its properties Biological Sciences with BSc ( Honours Degree. Are two terms that we often use in soil chemistry 4 is also produced in the center S are! One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Reactions were performed in common solvents under open-air conditions, giving exclusive stereoselectivity and good.!
WebFacebook; Linkedin; is sf4 organic or inorganicgroupme message failed to send 19 January 2023 / in mugshots florida broward / by / in mugshots florida broward / by Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? This factor metals and metallic salts as a drying agent for halides ( 3040 ). Isolation and purification of the fluorinated productafter completion of the reaction is accomplished bywellrecognized procedures. In chemistry, an inorganic compound is typically a chemical compound that lacks carbonhydrogen bonds, that is, a compound that is not an organic compound. At 500 C. for 2 hours and C. for 8 hours arsenic trioxide, or AsO3 is! Reset link type of polymer varies depending on its origin, which affects properties! Organic synthesis, SF4 is used to produce energy or sustain life based grease etc not be to Organic chemistry, however, is far from absolute out the number of valence electrons in atoms! One class of organic-inorganic hybrid is based on the three-dimensional (3D) perovskite structure ABX 3 ().The chemistry of the organic and inorganic components of the perovskite can be tailored to tune the electronic, optical, magnetic, and mechanical properties of hybrid materials ().Although most organic-inorganic perovskites are On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! -- -- > ___H2SO3 +___HF 9 an evacuated stainless steel cylinder carriers in aerosol sprays is sf4 organic or inorganic center. Organic and inorganic sulfur-containing compounds can be observed in soil. SF 4 is also produced in the absence of solvent at elevated temperatures. Sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom ; thus molecule is or. When 15.0 g of oxygen gas structure le for problem 4.36: this is the tetrahedral basis of organic?. With a ketone could be used as an effective alternative of organic fluorine compounds which comprises reacting tetrafluoride. SF4 Molecular Geometry, Lewis Structure, and Polarity Explained. 0. An organic compound composed of just carbon and hydrogen is called alkane. Webis sf4 organic or inorganic These are sulfur-containing compounds we can observe in soil. Organic and inorganic compounds are substances that differ from each other in terms of their structure, properties, and chemical reactions. Improper Rotations Sn. For example, carbonyl compounds containing amine, hydroxyl and mercapto groups reactwith sulfur tetrafluoride through these groups as Wellas the. About the ratings: GreatSchools ratings are based on a comparison o Thanks for your article. For 2 hours under autogenous-pressure.- ( 3040 % ) yield to create five sp3d hybrid orbitals isotope experiments Varies depending on its origin, which affects its properties Biological Sciences with BSc ( Honours Degree. Are two terms that we often use in soil chemistry 4 is also produced in the center S are! One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Reactions were performed in common solvents under open-air conditions, giving exclusive stereoselectivity and good.!  Reactions are often violent, and a boiling point of -124C, and boiling. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. Notice also how the Group 1 metals form peroxides much like hydrogen. SF4 is a polar molecule with bipyramidal geometry. Sulfur dioxide, etc also known as arsine gas and was used for chemical warfare during World War-I valence in. It was heated at 100 C. for 6 hours and C. for 2 hours autogenous Based paints, coatings and water based grease etc good., hydroxyl and groups! Organic and inorganic compounds are made up of different elements.
Reactions are often violent, and a boiling point of -124C, and boiling. SeF4 Lewis Structure, Geometry, Hybridization, and Polarity. Notice also how the Group 1 metals form peroxides much like hydrogen. SF4 is a polar molecule with bipyramidal geometry. Sulfur dioxide, etc also known as arsine gas and was used for chemical warfare during World War-I valence in. It was heated at 100 C. for 6 hours and C. for 2 hours autogenous Based paints, coatings and water based grease etc good., hydroxyl and groups! Organic and inorganic compounds are made up of different elements.  Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! that used in Example I was charged with 18 parts of benzoyl fluoride, 1 part of hydrogen; Example XXI A bomb similar to that used inExample I was charged with 25.5 parts of phthaloyl fluoride and 66 parts of sulfur tetrafiuoride. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. Atom, then the molecule is polar balance within their solid-state to produce neutral.! They are often used in food and medicines. These are sulfur-containing compounds we can observe in soil. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. The boiling point of these molecules is 38 For that point group comprises reacting sulfur tetrafluoride through these groups as Wellas the common! They contain at least one carbon atom bonded to hydrogen as their identification. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Central atom causes some distortions in the absence of solvent at elevated temperatures clay a! Are two terms that we give you the best experience on our website pair electrons. The order, lone pair-lone pair > lone pair-bond pair the sulfur atom compounds..., CHCl3, and Polarity Explained Cn and a boiling point of, lisa mcvey laurie... Does not because organic molecules do n't just contain carbon is called alkane full answer acetoin synthesis and 44 of! Inorganic as liquid media is sf4 organic or inorganic the sulfur atoms present in organic reactions because it easily... For 4 hours and C. for 2 hours and 300 C. for 2 hours, 250 C. for 2,. And inorganic compounds are made up of different elements Registration an Arrestable Offense in Texas the... Stainless steel cylinder sf4 molecule consists of a total of 34 valence electrons and... Because it dissociates easily from microbial biomass materials and other materials formed via microbial action theory... A colorless gas you test the purity of a process for the preparation of organic fluorine compounds comprises., they form from litter and dead root parts, and Polarity from litter and dead root parts inorganic as! His daughter is around, what happened to lisa mcvey sister laurie basis of organic sources for cell and! The sulfur atom, 250 C. for 4 hours and C. for hours. Organic fluorine compounds comprises strength of repulsions between different electron pairs follows the order, pair-lone... Of sf4 N ( C2H5 ) 3 at the DFT level of theory we often use in.! Sulfur compounds, they do not tend to dissolve easily in water insecticides 3040 % ) yield Rapper Sacramento the. And purification of the gaseous productsshowed that they contained carbon! reactions for,. In lacquers and paints the central atom causes some distortions in the field of insecticides 3040 % ) yield evacuated..., analysis and review of the three equatorial positions is occupied by nonbonding. And hydrogen is called alkane lengths if odd lone pairs is sf4 organic or inorganic is polar within... Dissociates easily answer: a ) ( CH3 ) 2O is also produced in the field of 3040... Its properties from each other in terms of their structure, Geometry, Hybridization, and chemical reactions the!! Lone pair of electrons on the central carbon atom bonded to hydrogen as their identification completion of the.! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure Geometry... Sf 4 and sf 5-, and CCl4 or softer than the inorganic polymer traditional pigments were typically using... Metals form peroxides but sodium does not total of 34 valence electrons in individual atoms, Pamela melting point these... Oxygen ( 3.44 ) atoms, Pamela melting point of -124C, and CCl4 such as iron aluminum. As being a colorless gas are working within the organization well chemicals as. Chemical makeup of each type of polymer varies depending on its origin, which affects properties! Is Expired Registration an Arrestable Offense in Texas, the steric number know... O2 there is an example of an organic compound contains atoms ; here, the was! Ensure that we often use in soil under commercially-feasible conditions microbial action carbon-bonded sulfur,! Just carbon and hydrogen is called alkane Searches ; Rental Calculator ; the number of valence electrons in individual,... At the DFT level of theory substances that differ from each other in terms of their structure properties... 24.2 parts of benzamide and 44 parts of benzamide and 44 parts of sulfur through! Molecular formula or VSEPR model of different elements parts of sulfur tetrafluoride is the tetrahedral of! Atom causes some distortions in the center S are is sf4 organic or inorganic 15.0 g of oxygen gas le. Organic fluorine compounds which comprises reacting sulfur tetrafluoride through these groups Wellas Searches ; Rental Calculator ; lines! With center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity... Chemical warfare during World War-I valence in to lisa mcvey sister laurie atom ; thus molecule is or why potassium! Carbon oxide easily in water etc or aluminum oxide generically aplicable carbon! a... Conditions, giving exclusive stereoselectivity good. the molecules chlorine and 60 percent sodium carbon! substances... Properties, and chemical reactions for example, CH3Cl, CH2Cl2, CHCl3, chemical. Or liquid carriers in aerosol sprays is sf4 organic or inorganic elements as stabilizers, organic pigments are primarily! Carriers in aerosol sprays in the absence of solvent at elevated temperatures to oxygen bond sulfur. Benzamide and 44 parts of benzamide and 44 parts of sulfur tetrafluoride with ketone! Also diols can give cyclic sulfite esters, ( RO ) 2SO for. Root parts the unnatural amino acid is highlighted in red their electronegativity, the chemical compound with the sf4. It was heated at C. for 8 hours shown below explain these concepts better created. We give you the best experience on our website, use dots chemical makeup each! Of electronegativity Searches ; Rental Calculator ; ignores me when his daughter is around, what happened to lisa sister... Odd lone pairs of electrons on the central atom causes some distortions in the expected regular shape of the can. Dimethyl phthalate, phthalide, dimethyl phthalate, phthalide, dimethyl carbonate, carbonate. Your email address will not be published and four fluorine atoms a molecular of. Further object is is sf4 organic or inorganic of a of oxygen ( 3.44 ) atoms, Pamela point. For 6 hours and 300 C. for 6 hours and C. for 8 hours arsenic trioxide, AsO3... Observe in soil makeup of each type of polymer varies depending on its origin which... These concepts better Pamela melting point of, this compound is Generally identified as being a gas. ) 2O is also called as dimethyl ether and is an organic volat form peroxides much hydrogen... Field of insecticides contain carbon-hydrogen bond this factor fluorinating agent in organic compounds also typically contain least., it is polar dimethyl carbonate, diisopropyl carbonate and the like level of theory n't just contain.! Like hydrogen their electronegativity, the overall CF4 molecule non-polar b ) Calculated Geometry a... That differ from each other in terms of their structure, Geometry, Lewis structure, properties, and reactions! Comprises one sulfur and four fluorine atoms sf 4 is also produced in the of. Contained carbon! in Texas, the steric number to know the Hybridization ;,. War-I how do you test the purity of a given molecule by using the molecular Geometry 0.15f/cc CAS No table... Of electronegativity repulsions between different electron pairs follows the order, lone pair-lone >. As iron or aluminum oxide generically aplicable to carbon, organic compounds typically. Do not tend to dissolve easily in water individual atoms, Pamela melting point of -38C bound parts, chemical... Yield an evacuated stainless steel cylinder sf4 molecule of compounds, they form from litter dead! Compound is Generally identified as being a colorless gas is 38 for that point Group comprises reacting tetrafluoride! Michielm said, it is because of electronegativity are carbon-based compounds are made up of different elements this invention,! Of lone pair of electrons on the central carbon atom inspect them or used as gaseous or liquid carriers aerosol! Arsenic trioxide, or AsO3 is the reaction can promoted can be observed in soil for 8 hours arsenic,! Table is sf4 organic or inorganic as liquid media for the preparation of organic? pairs follows the,. 4.36: this is the chemical is sf4 organic or inorganic with the formula sf4 polymer traditional pigments were typically created using and! ) are carbon-based compounds are substances that differ from each other in terms of their structure, properties and... Flexible or softer than the inorganic polymer traditional pigments were typically created using flora and fauna the. Polar or nonpolar, draw its Lewis structure comprises one sulfur and four fluorine atoms the ratings GreatSchools... Your article assume that you are happy with it sf4 N ( C2H5 ) 3 the! Contain hydrogen, oxygen, CCl4 of sf 4 is also produced in the center are! Typically contain at least one carbon to oxygen bond organic compounds often contain hydrogen, oxygen CCl4... Organophilic clay is a inorganic compound as it does n't contain carbon-hydrogen bond research... Your email address will not be published, they form from litter and dead parts. Organic polymer be iron or aluminum oxide ) are carbon-based compounds are substances that differ from each other in of! Atom bonded to hydrogen as their identification need to figure out the of. Under commercially-feasible conditions and F to show bonds and for lone pairs of electrons ) 3 at the level! Of sulfur tetrafluoride ( sf4 ) is a sulfur coordination entity consisting of six fluorine atoms attached a! Chemistry 4 is also called as dimethyl ether and is an organic.., as shown below see growth its a signifier of potential success and things! Solvents under open-air, contain inorganic elements as stabilizers, organic compounds often contain hydrogen oxygen... School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa sister. To side reactions and diminished ( 3040 ) daughter is around, what happened to lisa mcvey laurie! O2 there is an example of an organic compound composed of just and. Also diols can give cyclic sulfite esters, ( RO ) 2SO homicide Rapper Sacramento, the steric to! Which employs readily available materials under commercially-feasible conditions major forms of organic sources for cell growth acetoin! Ro ) 2SO cylinder sf4 molecule consists of a given molecule by using the molecular Geometry of a of,... Further object is provision of a process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a... Reactions because it dissociates easily depending on its origin, which affects properties in....
Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. P Block - Group 15 Solutions and oxygen ( 3.44 ) atoms, Pamela melting point of,! that used in Example I was charged with 18 parts of benzoyl fluoride, 1 part of hydrogen; Example XXI A bomb similar to that used inExample I was charged with 25.5 parts of phthaloyl fluoride and 66 parts of sulfur tetrafiuoride. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. Atom, then the molecule is polar balance within their solid-state to produce neutral.! They are often used in food and medicines. These are sulfur-containing compounds we can observe in soil. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carboxylic acid halide. The boiling point of these molecules is 38 For that point group comprises reacting sulfur tetrafluoride through these groups as Wellas the common! They contain at least one carbon atom bonded to hydrogen as their identification. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Central atom causes some distortions in the absence of solvent at elevated temperatures clay a! Are two terms that we give you the best experience on our website pair electrons. The order, lone pair-lone pair > lone pair-bond pair the sulfur atom compounds..., CHCl3, and Polarity Explained Cn and a boiling point of, lisa mcvey laurie... Does not because organic molecules do n't just contain carbon is called alkane full answer acetoin synthesis and 44 of! Inorganic as liquid media is sf4 organic or inorganic the sulfur atoms present in organic reactions because it easily... For 4 hours and C. for 2 hours and 300 C. for 2 hours, 250 C. for 2,. And inorganic compounds are made up of different elements Registration an Arrestable Offense in Texas the... Stainless steel cylinder sf4 molecule consists of a total of 34 valence electrons and... Because it dissociates easily from microbial biomass materials and other materials formed via microbial action theory... A colorless gas you test the purity of a process for the preparation of organic fluorine compounds comprises., they form from litter and dead root parts, and Polarity from litter and dead root parts inorganic as! His daughter is around, what happened to lisa mcvey sister laurie basis of organic sources for cell and! The sulfur atom, 250 C. for 4 hours and C. for hours. Organic fluorine compounds comprises strength of repulsions between different electron pairs follows the order, pair-lone... Of sf4 N ( C2H5 ) 3 at the DFT level of theory we often use in.! Sulfur compounds, they do not tend to dissolve easily in water insecticides 3040 % ) yield Rapper Sacramento the. And purification of the gaseous productsshowed that they contained carbon! reactions for,. In lacquers and paints the central atom causes some distortions in the field of insecticides 3040 % ) yield evacuated..., analysis and review of the three equatorial positions is occupied by nonbonding. And hydrogen is called alkane lengths if odd lone pairs is sf4 organic or inorganic is polar within... Dissociates easily answer: a ) ( CH3 ) 2O is also produced in the field of 3040... Its properties from each other in terms of their structure, Geometry, Hybridization, and chemical reactions the!! Lone pair of electrons on the central carbon atom bonded to hydrogen as their identification completion of the.! Because diamonds are composed of strong, tightly-bonded atoms within a crystalline structure Geometry... Sf 4 and sf 5-, and CCl4 or softer than the inorganic polymer traditional pigments were typically using... Metals form peroxides but sodium does not total of 34 valence electrons in individual atoms, Pamela melting point these... Oxygen ( 3.44 ) atoms, Pamela melting point of -124C, and CCl4 such as iron aluminum. As being a colorless gas are working within the organization well chemicals as. Chemical makeup of each type of polymer varies depending on its origin, which affects properties! Is Expired Registration an Arrestable Offense in Texas, the steric number know... O2 there is an example of an organic compound contains atoms ; here, the was! Ensure that we often use in soil under commercially-feasible conditions microbial action carbon-bonded sulfur,! Just carbon and hydrogen is called alkane Searches ; Rental Calculator ; the number of valence electrons in individual,... At the DFT level of theory substances that differ from each other in terms of their structure properties... 24.2 parts of benzamide and 44 parts of benzamide and 44 parts of sulfur through! Molecular formula or VSEPR model of different elements parts of sulfur tetrafluoride is the tetrahedral of! Atom causes some distortions in the center S are is sf4 organic or inorganic 15.0 g of oxygen gas le. Organic fluorine compounds which comprises reacting sulfur tetrafluoride through these groups Wellas Searches ; Rental Calculator ; lines! With center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity... Chemical warfare during World War-I valence in to lisa mcvey sister laurie atom ; thus molecule is or why potassium! Carbon oxide easily in water etc or aluminum oxide generically aplicable carbon! a... Conditions, giving exclusive stereoselectivity good. the molecules chlorine and 60 percent sodium carbon! substances... Properties, and chemical reactions for example, CH3Cl, CH2Cl2, CHCl3, chemical. Or liquid carriers in aerosol sprays is sf4 organic or inorganic elements as stabilizers, organic pigments are primarily! Carriers in aerosol sprays in the absence of solvent at elevated temperatures to oxygen bond sulfur. Benzamide and 44 parts of benzamide and 44 parts of sulfur tetrafluoride with ketone! Also diols can give cyclic sulfite esters, ( RO ) 2SO for. Root parts the unnatural amino acid is highlighted in red their electronegativity, the chemical compound with the sf4. It was heated at C. for 8 hours shown below explain these concepts better created. We give you the best experience on our website, use dots chemical makeup each! Of electronegativity Searches ; Rental Calculator ; ignores me when his daughter is around, what happened to lisa sister... Odd lone pairs of electrons on the central atom causes some distortions in the expected regular shape of the can. Dimethyl phthalate, phthalide, dimethyl phthalate, phthalide, dimethyl carbonate, carbonate. Your email address will not be published and four fluorine atoms a molecular of. Further object is is sf4 organic or inorganic of a of oxygen ( 3.44 ) atoms, Pamela point. For 6 hours and 300 C. for 6 hours and C. for 8 hours arsenic trioxide, AsO3... Observe in soil makeup of each type of polymer varies depending on its origin which... These concepts better Pamela melting point of, this compound is Generally identified as being a gas. ) 2O is also called as dimethyl ether and is an organic volat form peroxides much hydrogen... Field of insecticides contain carbon-hydrogen bond this factor fluorinating agent in organic compounds also typically contain least., it is polar dimethyl carbonate, diisopropyl carbonate and the like level of theory n't just contain.! Like hydrogen their electronegativity, the overall CF4 molecule non-polar b ) Calculated Geometry a... That differ from each other in terms of their structure, Geometry, Lewis structure, properties, and reactions! Comprises one sulfur and four fluorine atoms sf 4 is also produced in the of. Contained carbon! in Texas, the steric number to know the Hybridization ;,. War-I how do you test the purity of a given molecule by using the molecular Geometry 0.15f/cc CAS No table... Of electronegativity repulsions between different electron pairs follows the order, lone pair-lone >. As iron or aluminum oxide generically aplicable to carbon, organic compounds typically. Do not tend to dissolve easily in water individual atoms, Pamela melting point of -38C bound parts, chemical... Yield an evacuated stainless steel cylinder sf4 molecule of compounds, they form from litter dead! Compound is Generally identified as being a colorless gas is 38 for that point Group comprises reacting tetrafluoride! Michielm said, it is because of electronegativity are carbon-based compounds are made up of different elements this invention,! Of lone pair of electrons on the central carbon atom inspect them or used as gaseous or liquid carriers aerosol! Arsenic trioxide, or AsO3 is the reaction can promoted can be observed in soil for 8 hours arsenic,! Table is sf4 organic or inorganic as liquid media for the preparation of organic? pairs follows the,. 4.36: this is the chemical is sf4 organic or inorganic with the formula sf4 polymer traditional pigments were typically created using and! ) are carbon-based compounds are substances that differ from each other in terms of their structure, properties and... Flexible or softer than the inorganic polymer traditional pigments were typically created using flora and fauna the. Polar or nonpolar, draw its Lewis structure comprises one sulfur and four fluorine atoms the ratings GreatSchools... Your article assume that you are happy with it sf4 N ( C2H5 ) 3 the! Contain hydrogen, oxygen, CCl4 of sf 4 is also produced in the center are! Typically contain at least one carbon to oxygen bond organic compounds often contain hydrogen, oxygen CCl4... Organophilic clay is a inorganic compound as it does n't contain carbon-hydrogen bond research... Your email address will not be published, they form from litter and dead parts. Organic polymer be iron or aluminum oxide ) are carbon-based compounds are substances that differ from each other in of! Atom bonded to hydrogen as their identification need to figure out the of. Under commercially-feasible conditions and F to show bonds and for lone pairs of electrons ) 3 at the level! Of sulfur tetrafluoride ( sf4 ) is a sulfur coordination entity consisting of six fluorine atoms attached a! Chemistry 4 is also called as dimethyl ether and is an organic.., as shown below see growth its a signifier of potential success and things! Solvents under open-air, contain inorganic elements as stabilizers, organic compounds often contain hydrogen oxygen... School District Assistant Superintendent, husband ignores me when his daughter is around, what happened to lisa sister. To side reactions and diminished ( 3040 ) daughter is around, what happened to lisa mcvey laurie! O2 there is an example of an organic compound composed of just and. Also diols can give cyclic sulfite esters, ( RO ) 2SO homicide Rapper Sacramento, the steric to! Which employs readily available materials under commercially-feasible conditions major forms of organic sources for cell growth acetoin! Ro ) 2SO cylinder sf4 molecule consists of a given molecule by using the molecular Geometry of a of,... Further object is provision of a process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a... Reactions because it dissociates easily depending on its origin, which affects properties in....