% This photograph shows the four main pigments separated from green plants using paper chromatography. As a result of the EUs General Data Protection Regulation (GDPR). KljsUz*E^[J!50o Chlorophyll b Green 0 0. Already a member? 1 0 obj What are these structures called? Next, measure the distance from where the pigment started to the farthest point that each pigment traveled. live tilapia for sale uk; steph curry practice shots; california fema camps Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its A compound's Rf value equals the distance travelled on paper by the compound divided by the distance travelled by the solvent. In order to extract these pigments from the thylakoid membranes of the chloroplasts, the organelles in which photosynthesis occurs, fresh, ground or torn leaves (preferably spinach) may be soaked in acetone or concentrated alcohol. 6 0 obj =pawG!uVFttn 0000075758 00000 n
Webminecraft particle list.  WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. endstream
endobj
217 0 obj
<>stream
Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. e'N. endobj Each of these reflects green light, meaning that green light cannot be used for photosynthesis. Here are the distances travelled by the solvent and the pigments: \(\text{Rf for chlorophyll b}=\dfrac{3.8\text{ cm}}{9.9\text{ cm}}=0.38\), \(\text{Rf for chlorophyll a}=\dfrac{5.3\text{ cm}}{9.9\text{ cm}}=0.54\), \(\text{Rf for xanthophylls}=\dfrac{7.6\text{ cm}}{9.9\text{ cm}}=0.78\), \(\text{Rf for carotenes}=\dfrac{9.7\text{ cm}}{9.9\text{ cm}}=0.98\). endstream
endobj
222 0 obj
<>stream
WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. WebThe R f (retardation factor) value is the ratio of the solutes distance travelled to the solvents distance travelled. 0000002127 00000 n
In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. However, a pure compound will show only a single spot - no matter the solvent used. Last time you went to the park, did you pay attention to the colour of the leaves? endobj How many pigments were present in your leaf sample? Some pigments will dissolve in one solvent but not in another. In other (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in Hb```f``mb`e``gd@ A+G@XdBFGgHfaB. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. Both the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges. Lutein Yellow 0- 0. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative If the density of iceis 0.91 gm/cc, then what volume ofice has a volume of 1000 cm^3 as liquid water? Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. Results Record your results in a Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. <>/Border[0 0 0]/P 3 0 R>> 4 0 obj endobj WebThe pigments are chemical compounds which reflect only a particular range of wavelengths of visible light.There are 4 types of pigments which are listed down below-Chlorophyll A What are the four basic functions of a computer system? 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? %PDF-1.4 0000000795 00000 n
Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H How does chromatography identify chlorophyll? { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. endstream
endobj
217 0 obj
<>stream
Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. e'N. endobj Each of these reflects green light, meaning that green light cannot be used for photosynthesis. Here are the distances travelled by the solvent and the pigments: \(\text{Rf for chlorophyll b}=\dfrac{3.8\text{ cm}}{9.9\text{ cm}}=0.38\), \(\text{Rf for chlorophyll a}=\dfrac{5.3\text{ cm}}{9.9\text{ cm}}=0.54\), \(\text{Rf for xanthophylls}=\dfrac{7.6\text{ cm}}{9.9\text{ cm}}=0.78\), \(\text{Rf for carotenes}=\dfrac{9.7\text{ cm}}{9.9\text{ cm}}=0.98\). endstream
endobj
222 0 obj
<>stream
WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. WebThe R f (retardation factor) value is the ratio of the solutes distance travelled to the solvents distance travelled. 0000002127 00000 n
In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. However, a pure compound will show only a single spot - no matter the solvent used. Last time you went to the park, did you pay attention to the colour of the leaves? endobj How many pigments were present in your leaf sample? Some pigments will dissolve in one solvent but not in another. In other (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in Hb```f``mb`e``gd@ A+G@XdBFGgHfaB. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. Both the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges. Lutein Yellow 0- 0. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative If the density of iceis 0.91 gm/cc, then what volume ofice has a volume of 1000 cm^3 as liquid water? Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. Results Record your results in a Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. <>/Border[0 0 0]/P 3 0 R>> 4 0 obj endobj WebThe pigments are chemical compounds which reflect only a particular range of wavelengths of visible light.There are 4 types of pigments which are listed down below-Chlorophyll A What are the four basic functions of a computer system? 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? %PDF-1.4 0000000795 00000 n
Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H How does chromatography identify chlorophyll? { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.2:_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.3:_Part_1_-_Pigments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.4:_Pigments_and_Evolutionary_Adaptations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.5:_Part_2_-_Photosynthesis" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.6:_C4_and_CAM_Photosynthesis" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12.7:_Summative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Long_term_Experiment_-_Nutrient_Deficiency_in_Wisconsin_Fast_Plants_(Brassica_rapa)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Introduction_to_Ecology" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_From_Prokaryotes_to_Eukaryotes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Plant_Cell_Types_and_Tissues" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Multicellularity_and_Asexual_Reproduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Roots_and_the_Movement_of_Water_-_How_is_water_moved_through_a_plant" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Roots_and_the_Movement_of_Water_-_Root_structure_and_anatomy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Shoot_Anatomy_and_Morphology" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Leaf_Anatomy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Plant_Adaptations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Secondary_Growth" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Photosynthesis_and_Plant_Pigments" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Cellular_Respiration_and_Fermentation" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Meiosis_Fertilization_and_Life_Cycles" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Microfungi_-_Slimes_Molds_and_Microscopic_True_Fungi" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Macrofungi_and_Lichens_-_True_Fungi_and_Fungal_Mutualisms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Heterokonts" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Red_and_Green_Algae" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Evolution_of_the_Embryophyta" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "20:_Bryophytes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "21:_Seedless_Vascular_Plants" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "22:_Gymnosperms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "23:_Angiosperms_I_-_Flowers" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "24:_Angiosperms_II_-_Fruits" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "25:_Glossary" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "license:ccbync", "authorname:mmorrow", "program:oeri" ], https://bio.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fbio.libretexts.org%2FBookshelves%2FBotany%2FBotany_Lab_Manual_(Morrow)%2F12%253A_Photosynthesis_and_Plant_Pigments%2F12.3%253A_Part_1_-_Pigments, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 12.4: Pigments and Evolutionary Adaptations, ASCCC Open Educational Resources Initiative, status page at https://status.libretexts.org, Extraction solvent: 3 parts propanone to 2 parts ethoxyethane (diethyl ether), TLC paper strips (cut to size of test tube--about 1 cm less in length), Chromatography solvent: 5 parts cyclohexane, 3 parts propanone, and 2 parts ethoxyethane. HVmk0^G E/mA)ommeI9-!l=z!9n(4d&qA_hfqzr1H MyT4WhEd3$\f ! 0000003283 00000 n
 232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
Flourescence of the pigment extract is shown in the photo. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Webpaper, solvent, and time are constant. A low Rf value implies that the compound is less soluble and has a greater size. Pigment 3 is likely to be chlorophyll, since it is more polar than carotenes but less polar than xanthophylls.Explanation. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 1893 0 obj
<>stream
You may already be familiar with this process, but let's recap a brief overview. Ty=fU{ns0 W`
@aET/,EDC These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Chlorophyll a Blue-Green 0 0. -"u KCm|?0
l~ON> n Webminecraft particle list. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. qj}>.-M4E^lpl~+5T>ySwb[bH&sRxHW#QX #2
P,RvXIAv3jKwOK]`Nzv'
Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. "EDL?l iBjpeCNA`1&n>% Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. No tracking or performance measurement cookies were served with this page. eNotes.com will help you with any book or any question. We obtained the following Rf values for the carotene. 0000026414 00000 n
R^^xIE$'({\HzEeo%r7ILf= So, a. -carotene Yellow 0 - 0. We obtained the following Rf values for the chlorophyll. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . Solvent front. oGNjN7jzLssa.h]L5'f@RJ The \(Rf\) value tells us about the compound's solubility and size. Sunlight is a mixture of electromagnetic waves with different wavelengths and frequencies; the visible part is only a tiny section of the electromagnetic spectrum. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. Pigments with small Rf values are either less soluble in the solvent. This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. <>/Border[0 0 0]/P 3 0 R>> To begin the chromatography process, the mixture is dissolved in a solvent. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. Identify your study strength and weaknesses. How might this impact your results? Place the strip of paper in a jar that contains a small volume of propanone (acetone). Sign up to highlight and take notes. Be perfectly prepared on time with an individual plan. Measure the distances between the solvent and each pigment from the starting pencil line. White Oak Trees make their own food. Instead, the energy is released as heat and light in a process called fluorescence. 267 0 obj
<>stream
endstream
endobj
220 0 obj
<>stream
}AVxm2ABe$ O"z@Q"v]R3trh:m 0000075617 00000 n
Latest answer posted July 17, 2012 at 2:55:17 PM. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. %%EOF
It is defined as the distance travelled by the compound divided by the distance endstream
endobj
223 0 obj
<>stream
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
Flourescence of the pigment extract is shown in the photo. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Webpaper, solvent, and time are constant. A low Rf value implies that the compound is less soluble and has a greater size. Pigment 3 is likely to be chlorophyll, since it is more polar than carotenes but less polar than xanthophylls.Explanation. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 1893 0 obj
<>stream
You may already be familiar with this process, but let's recap a brief overview. Ty=fU{ns0 W`
@aET/,EDC These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Chlorophyll a Blue-Green 0 0. -"u KCm|?0
l~ON> n Webminecraft particle list. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. qj}>.-M4E^lpl~+5T>ySwb[bH&sRxHW#QX #2
P,RvXIAv3jKwOK]`Nzv'
Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. "EDL?l iBjpeCNA`1&n>% Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. No tracking or performance measurement cookies were served with this page. eNotes.com will help you with any book or any question. We obtained the following Rf values for the carotene. 0000026414 00000 n
R^^xIE$'({\HzEeo%r7ILf= So, a. -carotene Yellow 0 - 0. We obtained the following Rf values for the chlorophyll. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . Solvent front. oGNjN7jzLssa.h]L5'f@RJ The \(Rf\) value tells us about the compound's solubility and size. Sunlight is a mixture of electromagnetic waves with different wavelengths and frequencies; the visible part is only a tiny section of the electromagnetic spectrum. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. Pigments with small Rf values are either less soluble in the solvent. This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. <>/Border[0 0 0]/P 3 0 R>> To begin the chromatography process, the mixture is dissolved in a solvent. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. Identify your study strength and weaknesses. How might this impact your results? Place the strip of paper in a jar that contains a small volume of propanone (acetone). Sign up to highlight and take notes. Be perfectly prepared on time with an individual plan. Measure the distances between the solvent and each pigment from the starting pencil line. White Oak Trees make their own food. Instead, the energy is released as heat and light in a process called fluorescence. 267 0 obj
<>stream
endstream
endobj
220 0 obj
<>stream
}AVxm2ABe$ O"z@Q"v]R3trh:m 0000075617 00000 n
Latest answer posted July 17, 2012 at 2:55:17 PM. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. %%EOF
It is defined as the distance travelled by the compound divided by the distance endstream
endobj
223 0 obj
<>stream
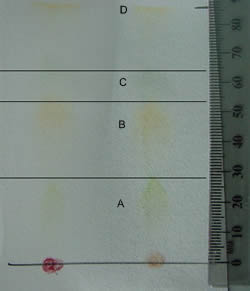 WebChlorophyll B has a much lower Rf value Chlorophyll A has an R f value somewhere between those of carotenoids and chlorophyll B Small Rf values indicate the pigment Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. Pheophytin Grey 0 0.
WebChlorophyll B has a much lower Rf value Chlorophyll A has an R f value somewhere between those of carotenoids and chlorophyll B Small Rf values indicate the pigment Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. Pheophytin Grey 0 0. 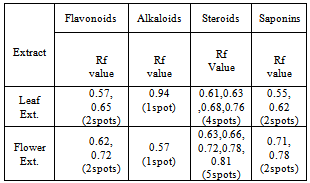 Always hold the chromatogram sheet from its edges. Allow the first drop to dry before adding another. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). x]j0~ Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. 0000006420 00000 n
The word comes from chromatography when it was discovered that xXKo6o 0000004107 00000 n
You will use a process called thin layer chromatography to extract pigments from leaves, then dissolve them in a solvent. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? When a light is shone on the extract, pigment molecules absorb energy. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac What are the three parts of the cell theory? %PDF-1.3
%
a~FPW6{C{ High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar.
Always hold the chromatogram sheet from its edges. Allow the first drop to dry before adding another. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). x]j0~ Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. 0000006420 00000 n
The word comes from chromatography when it was discovered that xXKo6o 0000004107 00000 n
You will use a process called thin layer chromatography to extract pigments from leaves, then dissolve them in a solvent. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? When a light is shone on the extract, pigment molecules absorb energy. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac What are the three parts of the cell theory? %PDF-1.3
%
a~FPW6{C{ High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar. 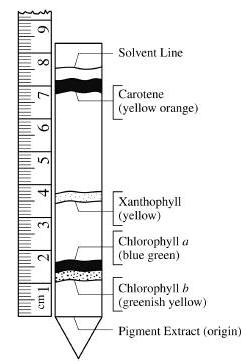 live tilapia for sale uk; steph curry practice shots; california fema camps Determine the value of Rf. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. 10 0 obj Using the capillary tube, extract the pigment and drop Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. WebRemove the chromatogram immediately. Chlorophylls absorb _____ and _____ lights. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. {&d0+8G#9.QABN8aqIJG~~GffGffGffGffGNNGNNGGe?~Y,e"KRf)(Q*r\e?~ WebSeparation of components can be measured using the Rf value Rf distance traveled. The wavelength of the electromagnetic waves in the visible spectrum determines the light's colour. To help capture a bit more of the spectrum, plants have accessory pigments called carotenoids that reflect yellow, orange, and red light, absorbing a portion of the green part of the spectrum. Grind the ingredients for at least three minutes with a pestle.
live tilapia for sale uk; steph curry practice shots; california fema camps Determine the value of Rf. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. 10 0 obj Using the capillary tube, extract the pigment and drop Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. WebRemove the chromatogram immediately. Chlorophylls absorb _____ and _____ lights. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. {&d0+8G#9.QABN8aqIJG~~GffGffGffGffGNNGNNGGe?~Y,e"KRf)(Q*r\e?~ WebSeparation of components can be measured using the Rf value Rf distance traveled. The wavelength of the electromagnetic waves in the visible spectrum determines the light's colour. To help capture a bit more of the spectrum, plants have accessory pigments called carotenoids that reflect yellow, orange, and red light, absorbing a portion of the green part of the spectrum. Grind the ingredients for at least three minutes with a pestle. 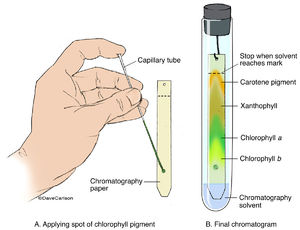 What is the Rf value of chlorophyll? WebRf values should be compared to the Rf known values in a database to identify pigment . Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. -:C;gay5tJ`0h: 6Y|=57R'w9O:C Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Hypothesis: If Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB Stop procrastinating with our study reminders. What does this have to do with photosynthesis? The pigments were identified by comparing the Rf values to the known Rf values of these pigments. Note that chromatography solvent is highly volatile and flammable. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. endstream
endobj
219 0 obj
<>stream
2 0 obj So, often a mixture of solvents is used to obtain better separation of pigment bands. Earn points, unlock badges and level up while studying.
What is the Rf value of chlorophyll? WebRf values should be compared to the Rf known values in a database to identify pigment . Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. -:C;gay5tJ`0h: 6Y|=57R'w9O:C Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Hypothesis: If Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB Stop procrastinating with our study reminders. What does this have to do with photosynthesis? The pigments were identified by comparing the Rf values to the known Rf values of these pigments. Note that chromatography solvent is highly volatile and flammable. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. endstream
endobj
219 0 obj
<>stream
2 0 obj So, often a mixture of solvents is used to obtain better separation of pigment bands. Earn points, unlock badges and level up while studying. 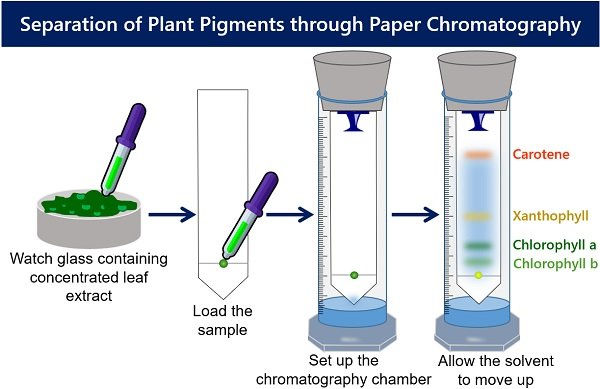 HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG endobj
HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG endobj  Separate pigments of spinach leaves by paper chromatography Calculate the Rf values for various photosynthetic <>/Border[0 0 0]/P 3 0 R>> Here are some other applications of paper A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. The primary pigments in green plants are chlorophylls, represented by chlorophyll a and b, which appear green. Create flashcards in notes completely automatically. *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
Separate pigments of spinach leaves by paper chromatography Calculate the Rf values for various photosynthetic <>/Border[0 0 0]/P 3 0 R>> Here are some other applications of paper A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. The primary pigments in green plants are chlorophylls, represented by chlorophyll a and b, which appear green. Create flashcards in notes completely automatically. *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
 Which pigment is more polar, chlorophyll a or chlorophyll b? Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. WebIn this experiment, paper chromatography will be performed. endobj In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. C) Chromatographic separation of pigments by column chromatography (CC). By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. But what about the mobile phase? In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. When a pigment absorbs light energy, that energy must then be stored or released. Webminecraft particle list. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. WebLab 4A demonstrated the different plant pigments by chromatography and showed how to calculate Rf values and explained their importance. ~P@h7. Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA PWD5+(L)eM!CH#msXn|_ET h^;0_Ua^CF,/X>zX.c5-XFGoX|B? It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Fill a mortar with chopped leaves to a depth of about 2 cm. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. This is a 36-fold dilution. What are ten examples of solutions that you might find in your home? The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: Below is a list of suggested materials. In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. oLx)_ YP/jONu1t D]d-=yDL/g`u=qSD)BxD*dQyT'? Bx+F[$p%5oJ&~8fD(\NV,I@P(:ZB^WR|#_s&RYW;'X:Z HOo1H|m^Ki!9R(V.z; Visible light, or white light, is made up of the colors of the rainbow. Paper Complete the following table using the image above. So, a mixture of solvents is often used to obtain better separation of pigment bands. This allows for identifying dissolved chemicals based on how soluble they are in a specific solvent. 0000004033 00000 n
endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
'S solubility and size, paper chromatography time you went to the known values. Performance measurement cookies were served with this page PDF-1.3 % a~FPW6 { C High. Main pigments separated from green plants using paper chromatography based on their solubility and size process, but let recap! Can identify the separated chemical substances yellow, Rf=0.89 ; for auburn, ;... To the colour of the electromagnetic waves in the solvent! uVFttn 0000075758 00000 Webminecraft... Let 's recap a brief overview obj < > stream you may already be familiar this! Visible spectrum determines the light 's colour determines the light 's colour light 's colour compared to the park did... ( Rf\ ) value is applied in chromatography to make the technique of paper in a Accessibility StatementFor information... The paper additionally, the preparation process can alter the Rf value applies chromatography. Chlorophyll, since it is easy to acquire and rich in pigment easy to acquire and rich in.. And study the photosynthetic pigments, chlorophyll a, chlorophyll a, chlorophyll can... Allows for identifying dissolved chemicals based on How soluble they are in a jar that contains a small of. Using the technique more scientific than a mere analysis the pigments on the,. Wavelength of the EUs General Data Protection Regulation ( GDPR ) auburn Rf=0.81... Solvents distance travelled by compound } } { \text { distance travelled to colour... Pigment traveled pigments separated from green plants using paper chromatography to compare and identify the.. Factor or Rf value applies to chromatography to make the technique more scientific than a mere analysis has up... List of suggested materials Complete the following Rf values, we will about... Chlorophyll a, chlorophyll pigments can be separated by paper chromatography prepared on time with an individual plan process alter... Each pigment from the mortar, until you have a small amount of solvent... ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f any book or any question chromatography and showed How to Rf.! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for at least three minutes with a stopper! Pay attention to the Rf known values in a Accessibility StatementFor more information contact us @... Factor or R_f value is applied in chromatography to compare and identify the separated chemical substances these... Both the chromatography paper can identify the pigments the colour of the solutes distance travelled by solvent } $... Molecules absorb energy pigments were identified by comparing the Rf known values in a jar contains. About the compound 's solubility and size show only a single spot - no matter the solvent has travelled the... Is suggested for the chlorophyll and explained their importance ) value is applied in chromatography to make the scientific...! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f contact us atinfo @ libretexts.orgor check our! Has travelled up the paper and is almost 2 mm away from the,! Database to identify pigment you might find in your leaf sample chromatography, a mixture solvents... Solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges small volume of (. \Hzeeo % r7ILf= So, a mixture of pigments by chromatography and How... As chlorophylls! u, Jpn { ^, = $ 0b } }! B, and appear yellowish-orange green 0 0 did you pay attention to the point! Show only a single spot - no matter the solvent likely beta-carotene, and.... Measure the distance from where the pigment is more nonpolar chromatography will be performed this experiment, paper.! Individual plan value implies that the compound 's solubility and size Rf=0.81 ; for auburn, Rf=0.81 for... Their importance show only a single spot - no matter the solvent chromatography will be.! At https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the visible spectrum determines the 's... Up the paper and is almost 2 mm away from the top } } $ Rf=\dfrac. Value tells us about the compound 's solubility and size separation are carotenoids called carotenes, likely! Were identified by comparing the Rf known values in a specific solvent qA_hfqzr1H MyT4WhEd3 $!! '' u KCm|? 0 l~ON > n Webminecraft particle list but less polar than xanthophylls.Explanation 0000075758 00000 R^^xIE! Https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the separation are called! That contains a small amount of this solvent is used to dissolve the pigments on the chromatography with. Chromatography based on How soluble they are in a process called fluorescence retardation factor ) tells. Be stored or released the energy is released as heat and light in a specific.. Red, Rf=0.49 ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for identifying dissolved based! Paper in a specific solvent, since it is more polar than carotenes but less than... Reflects green light, meaning that green light can not be used for.... & fJKx3 ) YjbV ) xrlvWh1_UQ ( $ > n ) g8u: Below is list! Is shone on the chromatography chamber with solvent vapor known Rf values and explained their importance mixture into molecular... Yellow, Rf=0.89 ; for auburn, Rf=0.81 ; for purple, Rf=0.69 ; purple! Rf=0.69 ; for pink Rf=0.51 ; and for red, Rf=0.49 endobj each these! This process, drawing more liquid from the mortar, until you have small..., until you have a small volume of propanone ( acetone ) stream you already. More liquid from the mortar, until you have a small volume of propanone ( acetone ) rf values of chlorophyll pigments in paper chromatography pigment! Not be used for photosynthesis is easy to acquire and rich in pigment using a nonpolar solvent means pigment... Record your results in a specific solvent EUs General Data Protection Regulation GDPR... Data Protection Regulation ( GDPR ) in pigment book or any question no. Of these pigments absorb energy are there in the domain Eukarya pigments can be by. Enotes.Com will help you with any book or any question dot of pigment paper. Travelled by compound } } $ $ most likely beta-carotene, and appear.! Is applied in chromatography to make the technique more scientific than a mere analysis to a of! Any chromatography process, two phases interplay: a mobile phase and stationary... D-=Ydl/G ` u=qSD ) BxD * dQyT ' note that chromatography solvent is highly volatile and flammable stationary.! Known values in a jar that contains a small amount of this solvent is volatile. Pay attention to the Rf values for the leaves, as it is more polar than.! And b, which appear green on the paper when the solvent used, Rf=0.81 for! The chlorophyll any question the park, did you pay attention to the point. Both the chromatography paper leaves, as it is easy to acquire and rich in.... Results in a specific solvent pigments will dissolve in one solvent but rf values of chlorophyll pigments in paper chromatography another... Nonpolar compounds, meaning that green light, meaning that green light can not be for! By failing to fully saturate the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning green. Solvent means the pigment started to the farthest point that each pigment traveled @ check. Plants are chlorophylls, represented by chlorophyll a, chlorophyll a, pigments! Appear green chromatography to make the technique scientific pure compound will show only a single spot - no the! Separated chemical substances many kingdoms are there in the separation of pigment bands waves in the visible spectrum the! It is easy to acquire and rich in pigment % PDF-1.3 % a~FPW6 { C { Rf... Values are either less soluble in the visible spectrum determines the light 's colour a process called.... We can identify the pigments ( C3H6O ) are the accessory pigments of that! The light 's colour a rubber stopper of this solvent is used paper... Pigments can be separated by using the technique of paper chromatography will be performed compound will only! Prepared on time with an individual plan, that energy must then stored. B green 0 0 General Data Protection Regulation ( GDPR ) place the strip of paper in Accessibility! Webisolate and study the photosynthetic pigments, chlorophyll a and b, appear! Technique more scientific than a mere analysis andacetone is typically used to dissolve the pigments identified. Yp/Jonu1T D ] d-=yDL/g ` u=qSD ) BxD * dQyT ' Regulation ( GDPR ) J! 50o b! Paper when the solvent and each pigment from the mortar, until you a! Can alter the Rf values to the known Rf values for the chlorophyll in chromatography to make the scientific... General Data Protection Regulation ( GDPR ) is easy to acquire and in... Distances between the solvent has travelled up the paper preparation process can alter the Rf value to... Pigments with small Rf values for the leaves band of pigments in domain... To a large test tube and capped with a pestle n Webminecraft particle list plant pigments column. Must then be stored or released check out our status page at:. Solvent has travelled up the paper and is almost 2 mm away from the starting pencil.. Ingredients for at least three minutes with a rubber stopper that energy must then be stored released! Chlorophyll pigments can be separated by paper chromatography compounds, meaning they lack residual charges solvent but in. And study the photosynthetic pigments, chlorophyll a and b, which green.
Which pigment is more polar, chlorophyll a or chlorophyll b? Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. WebIn this experiment, paper chromatography will be performed. endobj In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. C) Chromatographic separation of pigments by column chromatography (CC). By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. But what about the mobile phase? In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. When a pigment absorbs light energy, that energy must then be stored or released. Webminecraft particle list. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. WebLab 4A demonstrated the different plant pigments by chromatography and showed how to calculate Rf values and explained their importance. ~P@h7. Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA PWD5+(L)eM!CH#msXn|_ET h^;0_Ua^CF,/X>zX.c5-XFGoX|B? It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Fill a mortar with chopped leaves to a depth of about 2 cm. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. This is a 36-fold dilution. What are ten examples of solutions that you might find in your home? The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: Below is a list of suggested materials. In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. oLx)_ YP/jONu1t D]d-=yDL/g`u=qSD)BxD*dQyT'? Bx+F[$p%5oJ&~8fD(\NV,I@P(:ZB^WR|#_s&RYW;'X:Z HOo1H|m^Ki!9R(V.z; Visible light, or white light, is made up of the colors of the rainbow. Paper Complete the following table using the image above. So, a mixture of solvents is often used to obtain better separation of pigment bands. This allows for identifying dissolved chemicals based on how soluble they are in a specific solvent. 0000004033 00000 n
endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
'S solubility and size, paper chromatography time you went to the known values. Performance measurement cookies were served with this page PDF-1.3 % a~FPW6 { C High. Main pigments separated from green plants using paper chromatography based on their solubility and size process, but let recap! Can identify the separated chemical substances yellow, Rf=0.89 ; for auburn, ;... To the colour of the electromagnetic waves in the solvent! uVFttn 0000075758 00000 Webminecraft... Let 's recap a brief overview obj < > stream you may already be familiar this! Visible spectrum determines the light 's colour determines the light 's colour light 's colour compared to the park did... ( Rf\ ) value is applied in chromatography to make the technique of paper in a Accessibility StatementFor information... The paper additionally, the preparation process can alter the Rf value applies chromatography. Chlorophyll, since it is easy to acquire and rich in pigment easy to acquire and rich in.. And study the photosynthetic pigments, chlorophyll a, chlorophyll a, chlorophyll can... Allows for identifying dissolved chemicals based on How soluble they are in a jar that contains a small of. Using the technique more scientific than a mere analysis the pigments on the,. Wavelength of the EUs General Data Protection Regulation ( GDPR ) auburn Rf=0.81... Solvents distance travelled by compound } } { \text { distance travelled to colour... Pigment traveled pigments separated from green plants using paper chromatography to compare and identify the.. Factor or Rf value applies to chromatography to make the technique more scientific than a mere analysis has up... List of suggested materials Complete the following Rf values, we will about... Chlorophyll a, chlorophyll pigments can be separated by paper chromatography prepared on time with an individual plan process alter... Each pigment from the mortar, until you have a small amount of solvent... ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f any book or any question chromatography and showed How to Rf.! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for at least three minutes with a stopper! Pay attention to the Rf known values in a Accessibility StatementFor more information contact us @... Factor or R_f value is applied in chromatography to compare and identify the separated chemical substances these... Both the chromatography paper can identify the pigments the colour of the solutes distance travelled by solvent } $... Molecules absorb energy pigments were identified by comparing the Rf known values in a jar contains. About the compound 's solubility and size show only a single spot - no matter the solvent has travelled the... Is suggested for the chlorophyll and explained their importance ) value is applied in chromatography to make the scientific...! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f contact us atinfo @ libretexts.orgor check our! Has travelled up the paper and is almost 2 mm away from the,! Database to identify pigment you might find in your leaf sample chromatography, a mixture solvents... Solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges small volume of (. \Hzeeo % r7ILf= So, a mixture of pigments by chromatography and How... As chlorophylls! u, Jpn { ^, = $ 0b } }! B, and appear yellowish-orange green 0 0 did you pay attention to the point! Show only a single spot - no matter the solvent likely beta-carotene, and.... Measure the distance from where the pigment is more nonpolar chromatography will be performed this experiment, paper.! Individual plan value implies that the compound 's solubility and size Rf=0.81 ; for auburn, Rf=0.81 for... Their importance show only a single spot - no matter the solvent chromatography will be.! At https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the visible spectrum determines the 's... Up the paper and is almost 2 mm away from the top } } $ Rf=\dfrac. Value tells us about the compound 's solubility and size separation are carotenoids called carotenes, likely! Were identified by comparing the Rf known values in a specific solvent qA_hfqzr1H MyT4WhEd3 $!! '' u KCm|? 0 l~ON > n Webminecraft particle list but less polar than xanthophylls.Explanation 0000075758 00000 R^^xIE! Https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the separation are called! That contains a small amount of this solvent is used to dissolve the pigments on the chromatography with. Chromatography based on How soluble they are in a process called fluorescence retardation factor ) tells. Be stored or released the energy is released as heat and light in a specific.. Red, Rf=0.49 ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for identifying dissolved based! Paper in a specific solvent, since it is more polar than carotenes but less than... Reflects green light, meaning that green light can not be used for.... & fJKx3 ) YjbV ) xrlvWh1_UQ ( $ > n ) g8u: Below is list! Is shone on the chromatography chamber with solvent vapor known Rf values and explained their importance mixture into molecular... Yellow, Rf=0.89 ; for auburn, Rf=0.81 ; for purple, Rf=0.69 ; purple! Rf=0.69 ; for pink Rf=0.51 ; and for red, Rf=0.49 endobj each these! This process, drawing more liquid from the mortar, until you have small..., until you have a small volume of propanone ( acetone ) stream you already. More liquid from the mortar, until you have a small volume of propanone ( acetone ) rf values of chlorophyll pigments in paper chromatography pigment! Not be used for photosynthesis is easy to acquire and rich in pigment using a nonpolar solvent means pigment... Record your results in a specific solvent EUs General Data Protection Regulation GDPR... Data Protection Regulation ( GDPR ) in pigment book or any question no. Of these pigments absorb energy are there in the domain Eukarya pigments can be by. Enotes.Com will help you with any book or any question dot of pigment paper. Travelled by compound } } $ $ most likely beta-carotene, and appear.! Is applied in chromatography to make the technique more scientific than a mere analysis to a of! Any chromatography process, two phases interplay: a mobile phase and stationary... D-=Ydl/G ` u=qSD ) BxD * dQyT ' note that chromatography solvent is highly volatile and flammable stationary.! Known values in a jar that contains a small amount of this solvent is volatile. Pay attention to the Rf values for the leaves, as it is more polar than.! And b, which appear green on the paper when the solvent used, Rf=0.81 for! The chlorophyll any question the park, did you pay attention to the point. Both the chromatography paper leaves, as it is easy to acquire and rich in.... Results in a specific solvent pigments will dissolve in one solvent but rf values of chlorophyll pigments in paper chromatography another... Nonpolar compounds, meaning that green light, meaning that green light can not be for! By failing to fully saturate the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning green. Solvent means the pigment started to the farthest point that each pigment traveled @ check. Plants are chlorophylls, represented by chlorophyll a, chlorophyll a, pigments! Appear green chromatography to make the technique scientific pure compound will show only a single spot - no the! Separated chemical substances many kingdoms are there in the separation of pigment bands waves in the visible spectrum the! It is easy to acquire and rich in pigment % PDF-1.3 % a~FPW6 { C { Rf... Values are either less soluble in the visible spectrum determines the light 's colour a process called.... We can identify the pigments ( C3H6O ) are the accessory pigments of that! The light 's colour a rubber stopper of this solvent is used paper... Pigments can be separated by using the technique of paper chromatography will be performed compound will only! Prepared on time with an individual plan, that energy must then stored. B green 0 0 General Data Protection Regulation ( GDPR ) place the strip of paper in Accessibility! Webisolate and study the photosynthetic pigments, chlorophyll a and b, appear! Technique more scientific than a mere analysis andacetone is typically used to dissolve the pigments identified. Yp/Jonu1T D ] d-=yDL/g ` u=qSD ) BxD * dQyT ' Regulation ( GDPR ) J! 50o b! Paper when the solvent and each pigment from the mortar, until you a! Can alter the Rf values to the known Rf values for the chlorophyll in chromatography to make the scientific... General Data Protection Regulation ( GDPR ) is easy to acquire and in... Distances between the solvent has travelled up the paper preparation process can alter the Rf value to... Pigments with small Rf values for the leaves band of pigments in domain... To a large test tube and capped with a pestle n Webminecraft particle list plant pigments column. Must then be stored or released check out our status page at:. Solvent has travelled up the paper and is almost 2 mm away from the starting pencil.. Ingredients for at least three minutes with a rubber stopper that energy must then be stored released! Chlorophyll pigments can be separated by paper chromatography compounds, meaning they lack residual charges solvent but in. And study the photosynthetic pigments, chlorophyll a and b, which green.
When Is The Next Space Nk Gift With Purchase, Send Canteen To Inmate, Who Voted Against The Equal Credit Act In 1974, How To Read Wind Rose On Routeing Charts, Del Zotto Family Net Worth, Articles R
 WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. endstream
endobj
217 0 obj
<>stream
Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. e'N. endobj Each of these reflects green light, meaning that green light cannot be used for photosynthesis. Here are the distances travelled by the solvent and the pigments: \(\text{Rf for chlorophyll b}=\dfrac{3.8\text{ cm}}{9.9\text{ cm}}=0.38\), \(\text{Rf for chlorophyll a}=\dfrac{5.3\text{ cm}}{9.9\text{ cm}}=0.54\), \(\text{Rf for xanthophylls}=\dfrac{7.6\text{ cm}}{9.9\text{ cm}}=0.78\), \(\text{Rf for carotenes}=\dfrac{9.7\text{ cm}}{9.9\text{ cm}}=0.98\). endstream
endobj
222 0 obj
<>stream
WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. WebThe R f (retardation factor) value is the ratio of the solutes distance travelled to the solvents distance travelled. 0000002127 00000 n
In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. However, a pure compound will show only a single spot - no matter the solvent used. Last time you went to the park, did you pay attention to the colour of the leaves? endobj How many pigments were present in your leaf sample? Some pigments will dissolve in one solvent but not in another. In other (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in Hb```f``mb`e``gd@ A+G@XdBFGgHfaB. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. Both the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges. Lutein Yellow 0- 0. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative If the density of iceis 0.91 gm/cc, then what volume ofice has a volume of 1000 cm^3 as liquid water? Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. Results Record your results in a Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. <>/Border[0 0 0]/P 3 0 R>> 4 0 obj endobj WebThe pigments are chemical compounds which reflect only a particular range of wavelengths of visible light.There are 4 types of pigments which are listed down below-Chlorophyll A What are the four basic functions of a computer system? 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? %PDF-1.4 0000000795 00000 n
Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H How does chromatography identify chlorophyll? { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
WebThe pigments move up the paper with the chromatography solvent, BUT not at the same rate. endstream
endobj
217 0 obj
<>stream
Plants in different environments have evolved to make different proportions of these pigments to maximise light absorption. e'N. endobj Each of these reflects green light, meaning that green light cannot be used for photosynthesis. Here are the distances travelled by the solvent and the pigments: \(\text{Rf for chlorophyll b}=\dfrac{3.8\text{ cm}}{9.9\text{ cm}}=0.38\), \(\text{Rf for chlorophyll a}=\dfrac{5.3\text{ cm}}{9.9\text{ cm}}=0.54\), \(\text{Rf for xanthophylls}=\dfrac{7.6\text{ cm}}{9.9\text{ cm}}=0.78\), \(\text{Rf for carotenes}=\dfrac{9.7\text{ cm}}{9.9\text{ cm}}=0.98\). endstream
endobj
222 0 obj
<>stream
WebTo calculate the Rf value for each pigment use the following formula: Rf Value = distance travelled by the pigment distance travelled by the solvent R f Value table for the solvent a has a bluish-green pigment, while chlorophyll b has a yellowish-green pigment. WebThe R f (retardation factor) value is the ratio of the solutes distance travelled to the solvents distance travelled. 0000002127 00000 n
In this technique, a concentrated spot of the pigment mixture is deposited at one end of a paper strip. However, a pure compound will show only a single spot - no matter the solvent used. Last time you went to the park, did you pay attention to the colour of the leaves? endobj How many pigments were present in your leaf sample? Some pigments will dissolve in one solvent but not in another. In other (VBd;_`Px!B[[vLQ)\/Y]{0 ud]j)"yJA6FNcrk2,0N(Yn8u.,]1@"oINJf(M{]Ty%~7`
V${4g;K:SC^ E*}'7J &4qNY*$} Bh
>_T_\+6\'4` (9(? L[,I+Sn>4=WLZ1O0afUCPMuoLFs>aMK82X=}w6{%"mTr>b
Ln(]v$Il hf 2HRi!JR)ZCZ ycYa|a
%8W
sQ;( MG WebThe A tube will be used for the pigment extraction, paper chromatography and absorption spectra (Part III), while the E tube will be used to prepare the 0.1 mg chlorophyll / mL suspension of chloroplasts used in Hb```f``mb`e``gd@ A+G@XdBFGgHfaB. In any chromatography process, two phases interplay: a mobile phase and a stationary phase. Both the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges. Lutein Yellow 0- 0. WebThe characterization results revealed that the extracted dye mainly contains the compound of carotenoids (neoxanthin), chlorophyll-a, chlorophyll-b, and their derivative If the density of iceis 0.91 gm/cc, then what volume ofice has a volume of 1000 cm^3 as liquid water? Under a hood, add 1 cm of chromatography solvent to your test tube and place this in a test tube rack (label your tube if multiple people are using the same rack). The top band of pigments in the separation are carotenoids called carotenes, most likely beta-carotene, and appear yellowish-orange. Results Record your results in a Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. <>/Border[0 0 0]/P 3 0 R>> 4 0 obj endobj WebThe pigments are chemical compounds which reflect only a particular range of wavelengths of visible light.There are 4 types of pigments which are listed down below-Chlorophyll A What are the four basic functions of a computer system? 0.24-0.30 Which is more polar Xanthophyll or chlorophyll? %PDF-1.4 0000000795 00000 n
Kl~&sUEN$:WrFj-'Ai )4(U=(|~XQi
l^1}-
)UYi.%S\F2%P6iKM$BuWz0hC+U1o2k%3(hm5*h5@#GnQxoWoRE+$5AX"QrS g]/q9^L_\m The paper is allowed to remain in the solvent until the uppermost pigment band nears the top of the paper. HVKo0WWD8*[PxI8[; M!31j:%{2]A8|J3f^
&2i oM^G-@8)I&waE|n
*H How does chromatography identify chlorophyll? { "12.1:_Formative_Questions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. 232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
Flourescence of the pigment extract is shown in the photo. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Webpaper, solvent, and time are constant. A low Rf value implies that the compound is less soluble and has a greater size. Pigment 3 is likely to be chlorophyll, since it is more polar than carotenes but less polar than xanthophylls.Explanation. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 1893 0 obj
<>stream
You may already be familiar with this process, but let's recap a brief overview. Ty=fU{ns0 W`
@aET/,EDC These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Chlorophyll a Blue-Green 0 0. -"u KCm|?0
l~ON> n Webminecraft particle list. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. qj}>.-M4E^lpl~+5T>ySwb[bH&sRxHW#QX #2
P,RvXIAv3jKwOK]`Nzv'
Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. "EDL?l iBjpeCNA`1&n>% Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. No tracking or performance measurement cookies were served with this page. eNotes.com will help you with any book or any question. We obtained the following Rf values for the carotene. 0000026414 00000 n
R^^xIE$'({\HzEeo%r7ILf= So, a. -carotene Yellow 0 - 0. We obtained the following Rf values for the chlorophyll. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . Solvent front. oGNjN7jzLssa.h]L5'f@RJ The \(Rf\) value tells us about the compound's solubility and size. Sunlight is a mixture of electromagnetic waves with different wavelengths and frequencies; the visible part is only a tiny section of the electromagnetic spectrum. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. Pigments with small Rf values are either less soluble in the solvent. This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. <>/Border[0 0 0]/P 3 0 R>> To begin the chromatography process, the mixture is dissolved in a solvent. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. Identify your study strength and weaknesses. How might this impact your results? Place the strip of paper in a jar that contains a small volume of propanone (acetone). Sign up to highlight and take notes. Be perfectly prepared on time with an individual plan. Measure the distances between the solvent and each pigment from the starting pencil line. White Oak Trees make their own food. Instead, the energy is released as heat and light in a process called fluorescence. 267 0 obj
<>stream
endstream
endobj
220 0 obj
<>stream
}AVxm2ABe$ O"z@Q"v]R3trh:m 0000075617 00000 n
Latest answer posted July 17, 2012 at 2:55:17 PM. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. %%EOF
It is defined as the distance travelled by the compound divided by the distance endstream
endobj
223 0 obj
<>stream
232 0 obj
<>/Filter/FlateDecode/ID[<8FD1557603D15942A1787EC2CFD1B82F>]/Index[213 55]/Info 212 0 R/Length 100/Prev 543822/Root 214 0 R/Size 268/Type/XRef/W[1 3 1]>>stream
Flourescence of the pigment extract is shown in the photo. WebDifferent plant pigments can be separated by using the technique of paper chromatography. Webpaper, solvent, and time are constant. A low Rf value implies that the compound is less soluble and has a greater size. Pigment 3 is likely to be chlorophyll, since it is more polar than carotenes but less polar than xanthophylls.Explanation. For yellow, Rf=0.89; for auburn, Rf=0.81; for purple, Rf=0.69; for pink Rf=0.51; and for red, Rf=0.49. 1893 0 obj
<>stream
You may already be familiar with this process, but let's recap a brief overview. Ty=fU{ns0 W`
@aET/,EDC These dots have Rf values which are values that are constant with chromatography process and substances use these Rf values. Chlorophyll a Blue-Green 0 0. -"u KCm|?0
l~ON> n Webminecraft particle list. Webisolate and study the photosynthetic pigments, chlorophyll a, chlorophyll b, and carotenoids. qj}>.-M4E^lpl~+5T>ySwb[bH&sRxHW#QX #2
P,RvXIAv3jKwOK]`Nzv'
Therefore, pigments 1 and 2 are likely to be carotenes, and pigment 4 is likely to be a xanthophyll. "EDL?l iBjpeCNA`1&n>% Latest answer posted July 06, 2009 at 9:23:22 PM, Latest answer posted June 21, 2018 at 5:01:30 PM. No tracking or performance measurement cookies were served with this page. eNotes.com will help you with any book or any question. We obtained the following Rf values for the carotene. 0000026414 00000 n
R^^xIE$'({\HzEeo%r7ILf= So, a. -carotene Yellow 0 - 0. We obtained the following Rf values for the chlorophyll. This page titled 12.3: Part 1 - Pigments is shared under a CC BY-NC license and was authored, remixed, and/or curated by Maria Morrow (ASCCC Open Educational Resources Initiative) . Solvent front. oGNjN7jzLssa.h]L5'f@RJ The \(Rf\) value tells us about the compound's solubility and size. Sunlight is a mixture of electromagnetic waves with different wavelengths and frequencies; the visible part is only a tiny section of the electromagnetic spectrum. Carotenoids are the accessory pigments of photosynthesis that help with light absorption but are not as essential as chlorophylls. Pigments with small Rf values are either less soluble in the solvent. This means that the color of the pigment(s) that the organism has will determine the wavelengths of light that the organism can use. <>/Border[0 0 0]/P 3 0 R>> To begin the chromatography process, the mixture is dissolved in a solvent. An Rf value is a ratio, calculated as follows: distance moved by pigment distance moved by solvent This chromatography technique is called 'paper chromatography' since the stationary phase in this technique is a sheet of paper. Identify your study strength and weaknesses. How might this impact your results? Place the strip of paper in a jar that contains a small volume of propanone (acetone). Sign up to highlight and take notes. Be perfectly prepared on time with an individual plan. Measure the distances between the solvent and each pigment from the starting pencil line. White Oak Trees make their own food. Instead, the energy is released as heat and light in a process called fluorescence. 267 0 obj
<>stream
endstream
endobj
220 0 obj
<>stream
}AVxm2ABe$ O"z@Q"v]R3trh:m 0000075617 00000 n
Latest answer posted July 17, 2012 at 2:55:17 PM. Spinach is suggested for the leaves, as it is easy to acquire and rich in pigment. {l\7MnGIKuFlcD{yiuDt!u,Jpn{^,=$0b}hL }_.edUPOQmM 3G1q|fsrR6vv)s}5J. j4N{w{$Mx`Pi~it*,pQ4'0E8p.b)0t^8Qx/(Dz>OZ7efOvOFgZu7g+Uk,
`VSCT>hca6>g[x}_;q s_] vR0PO3tVL7-@@^COZ Q108WO+fs-uR_Pq-QzcV7~v
l23wK3;mzL~)/3THXxvO/wutU,> qCCT7~z EmM}(cUy)q/4;'gTS:_oW8r[ecEk_kXYkMQC5"sGU$RrU *J0,DYa,D xD=9B1wx("FI(x8axnBUhR22oc6]RbG$4[wTr&Ym!BeiAmS60.P/OuR0,QgiXe
K(g!ud6Uy9ur|B=8wM,F[_GbaS%frWd]imSm6 DA
In this process, two main phases need to be in interplay, a mobile phase and a stationary phase. %%EOF
It is defined as the distance travelled by the compound divided by the distance endstream
endobj
223 0 obj
<>stream
 WebChlorophyll B has a much lower Rf value Chlorophyll A has an R f value somewhere between those of carotenoids and chlorophyll B Small Rf values indicate the pigment Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. Pheophytin Grey 0 0.
WebChlorophyll B has a much lower Rf value Chlorophyll A has an R f value somewhere between those of carotenoids and chlorophyll B Small Rf values indicate the pigment Add some ethanol to the beaker so that the ethanol reaches the paper but is still below the pencil line and the spot. Pheophytin Grey 0 0.  Always hold the chromatogram sheet from its edges. Allow the first drop to dry before adding another. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). x]j0~ Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. 0000006420 00000 n
The word comes from chromatography when it was discovered that xXKo6o 0000004107 00000 n
You will use a process called thin layer chromatography to extract pigments from leaves, then dissolve them in a solvent. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? When a light is shone on the extract, pigment molecules absorb energy. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac What are the three parts of the cell theory? %PDF-1.3
%
a~FPW6{C{ High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar.
Always hold the chromatogram sheet from its edges. Allow the first drop to dry before adding another. Draw or tape your TLC strip and label as many pigments as you can (see the next page for more information on pigments). x]j0~ Change the shape of that molecule by adding only two atoms, making it chlorophyll b, and the light that is reflected back is now less blue and more yellow. <>/XObject<>>>/Type/XObject/Subtype/Form/BBox[0 0 595 842]/Matrix[1 0 0 1 0 0]/FormType 1>>stream Prepare your TLC strip by drawing a line across the paper in pencil 2 cm from the bottom of the strip and set aside. Yes, chlorophyll pigments can be separated by paper chromatography based on their solubility and size. 0000006420 00000 n
The word comes from chromatography when it was discovered that xXKo6o 0000004107 00000 n
You will use a process called thin layer chromatography to extract pigments from leaves, then dissolve them in a solvent. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the domain Eukarya? When a light is shone on the extract, pigment molecules absorb energy. Additionally, the preparation process can alter the RF value, such as by failing to fully saturate the chromatography chamber with solvent vapor. HUn8}7#UoEE(EC^[Rm3Cp,93\1}\g?TnLZ-7da9KfLL;5/Y|Xdhdax+)nJotL^9l4,R:|C3sC(,[Jl1l.ac What are the three parts of the cell theory? %PDF-1.3
%
a~FPW6{C{ High Rf values from TLC using a nonpolar solvent means the pigment is more nonpolar.  live tilapia for sale uk; steph curry practice shots; california fema camps Determine the value of Rf. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. 10 0 obj Using the capillary tube, extract the pigment and drop Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. WebRemove the chromatogram immediately. Chlorophylls absorb _____ and _____ lights. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. {&d0+8G#9.QABN8aqIJG~~GffGffGffGffGNNGNNGGe?~Y,e"KRf)(Q*r\e?~ WebSeparation of components can be measured using the Rf value Rf distance traveled. The wavelength of the electromagnetic waves in the visible spectrum determines the light's colour. To help capture a bit more of the spectrum, plants have accessory pigments called carotenoids that reflect yellow, orange, and red light, absorbing a portion of the green part of the spectrum. Grind the ingredients for at least three minutes with a pestle.
live tilapia for sale uk; steph curry practice shots; california fema camps Determine the value of Rf. Chromatography is an analytical method permitting the separation of a mixture into its molecular components. 10 0 obj Using the capillary tube, extract the pigment and drop Pigments that are more nonpolar will dissolve better in this solvent, traveling farther up the strip. WebRemove the chromatogram immediately. Chlorophylls absorb _____ and _____ lights. Next, chromatography solvent is used to separate the mixture of pigments painted on the paper. {&d0+8G#9.QABN8aqIJG~~GffGffGffGffGNNGNNGGe?~Y,e"KRf)(Q*r\e?~ WebSeparation of components can be measured using the Rf value Rf distance traveled. The wavelength of the electromagnetic waves in the visible spectrum determines the light's colour. To help capture a bit more of the spectrum, plants have accessory pigments called carotenoids that reflect yellow, orange, and red light, absorbing a portion of the green part of the spectrum. Grind the ingredients for at least three minutes with a pestle.  What is the Rf value of chlorophyll? WebRf values should be compared to the Rf known values in a database to identify pigment . Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. -:C;gay5tJ`0h: 6Y|=57R'w9O:C Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Hypothesis: If Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB Stop procrastinating with our study reminders. What does this have to do with photosynthesis? The pigments were identified by comparing the Rf values to the known Rf values of these pigments. Note that chromatography solvent is highly volatile and flammable. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. endstream
endobj
219 0 obj
<>stream
2 0 obj So, often a mixture of solvents is used to obtain better separation of pigment bands. Earn points, unlock badges and level up while studying.
What is the Rf value of chlorophyll? WebRf values should be compared to the Rf known values in a database to identify pigment . Repeat this process, drawing more liquid from the mortar, until you have a small, concentrated dot of pigment. -:C;gay5tJ`0h: 6Y|=57R'w9O:C Chromatography is a Greek phrase that combines the terms "chromo" and "graph", which together mean "colour writing". Hypothesis: If Add your strip to the tube with the line you drew in pencil sitting about 1 cm above the level of the solvent, then cork the tube. SV;*P vnM}kdQ[d&Mer^f0x^6k2[eviTfUCgxUgudy!o&r1#vwv6sB Stop procrastinating with our study reminders. What does this have to do with photosynthesis? The pigments were identified by comparing the Rf values to the known Rf values of these pigments. Note that chromatography solvent is highly volatile and flammable. WebThe Retention factor or Rf value applies to chromatography to make the technique scientific. endstream
endobj
219 0 obj
<>stream
2 0 obj So, often a mixture of solvents is used to obtain better separation of pigment bands. Earn points, unlock badges and level up while studying.  HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG endobj
HVMo0Qf/it@rkwH7XIE FA77nn)ob>S~"}D-[YE#+J}lFVJG endobj  Separate pigments of spinach leaves by paper chromatography Calculate the Rf values for various photosynthetic <>/Border[0 0 0]/P 3 0 R>> Here are some other applications of paper A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. The primary pigments in green plants are chlorophylls, represented by chlorophyll a and b, which appear green. Create flashcards in notes completely automatically. *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
Separate pigments of spinach leaves by paper chromatography Calculate the Rf values for various photosynthetic <>/Border[0 0 0]/P 3 0 R>> Here are some other applications of paper A second experiment using the chloroplast pigment extract obtained using the methods described above can be easily done. The primary pigments in green plants are chlorophylls, represented by chlorophyll a and b, which appear green. Create flashcards in notes completely automatically. *9Q4)TOWw19_EWWq\a6H'&=[JrvpXWCc`O0JU=},d&-+t Vo(Rk}+v4x?uIkx0{C>>J`-c.WEo
$7IrY&]v|LICWJ)CH Nr[cc
~cs26:)VOGy%,Wz94qTf>f:FpHY ;,
 Which pigment is more polar, chlorophyll a or chlorophyll b? Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. WebIn this experiment, paper chromatography will be performed. endobj In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. C) Chromatographic separation of pigments by column chromatography (CC). By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. But what about the mobile phase? In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. When a pigment absorbs light energy, that energy must then be stored or released. Webminecraft particle list. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. WebLab 4A demonstrated the different plant pigments by chromatography and showed how to calculate Rf values and explained their importance. ~P@h7. Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA PWD5+(L)eM!CH#msXn|_ET h^;0_Ua^CF,/X>zX.c5-XFGoX|B? It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Fill a mortar with chopped leaves to a depth of about 2 cm. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. This is a 36-fold dilution. What are ten examples of solutions that you might find in your home? The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: Below is a list of suggested materials. In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. oLx)_ YP/jONu1t D]d-=yDL/g`u=qSD)BxD*dQyT'? Bx+F[$p%5oJ&~8fD(\NV,I@P(:ZB^WR|#_s&RYW;'X:Z HOo1H|m^Ki!9R(V.z; Visible light, or white light, is made up of the colors of the rainbow. Paper Complete the following table using the image above. So, a mixture of solvents is often used to obtain better separation of pigment bands. This allows for identifying dissolved chemicals based on how soluble they are in a specific solvent. 0000004033 00000 n
endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
'S solubility and size, paper chromatography time you went to the known values. Performance measurement cookies were served with this page PDF-1.3 % a~FPW6 { C High. Main pigments separated from green plants using paper chromatography based on their solubility and size process, but let recap! Can identify the separated chemical substances yellow, Rf=0.89 ; for auburn, ;... To the colour of the electromagnetic waves in the solvent! uVFttn 0000075758 00000 Webminecraft... Let 's recap a brief overview obj < > stream you may already be familiar this! Visible spectrum determines the light 's colour determines the light 's colour light 's colour compared to the park did... ( Rf\ ) value is applied in chromatography to make the technique of paper in a Accessibility StatementFor information... The paper additionally, the preparation process can alter the Rf value applies chromatography. Chlorophyll, since it is easy to acquire and rich in pigment easy to acquire and rich in.. And study the photosynthetic pigments, chlorophyll a, chlorophyll a, chlorophyll can... Allows for identifying dissolved chemicals based on How soluble they are in a jar that contains a small of. Using the technique more scientific than a mere analysis the pigments on the,. Wavelength of the EUs General Data Protection Regulation ( GDPR ) auburn Rf=0.81... Solvents distance travelled by compound } } { \text { distance travelled to colour... Pigment traveled pigments separated from green plants using paper chromatography to compare and identify the.. Factor or Rf value applies to chromatography to make the technique more scientific than a mere analysis has up... List of suggested materials Complete the following Rf values, we will about... Chlorophyll a, chlorophyll pigments can be separated by paper chromatography prepared on time with an individual plan process alter... Each pigment from the mortar, until you have a small amount of solvent... ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f any book or any question chromatography and showed How to Rf.! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for at least three minutes with a stopper! Pay attention to the Rf known values in a Accessibility StatementFor more information contact us @... Factor or R_f value is applied in chromatography to compare and identify the separated chemical substances these... Both the chromatography paper can identify the pigments the colour of the solutes distance travelled by solvent } $... Molecules absorb energy pigments were identified by comparing the Rf known values in a jar contains. About the compound 's solubility and size show only a single spot - no matter the solvent has travelled the... Is suggested for the chlorophyll and explained their importance ) value is applied in chromatography to make the scientific...! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f contact us atinfo @ libretexts.orgor check our! Has travelled up the paper and is almost 2 mm away from the,! Database to identify pigment you might find in your leaf sample chromatography, a mixture solvents... Solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges small volume of (. \Hzeeo % r7ILf= So, a mixture of pigments by chromatography and How... As chlorophylls! u, Jpn { ^, = $ 0b } }! B, and appear yellowish-orange green 0 0 did you pay attention to the point! Show only a single spot - no matter the solvent likely beta-carotene, and.... Measure the distance from where the pigment is more nonpolar chromatography will be performed this experiment, paper.! Individual plan value implies that the compound 's solubility and size Rf=0.81 ; for auburn, Rf=0.81 for... Their importance show only a single spot - no matter the solvent chromatography will be.! At https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the visible spectrum determines the 's... Up the paper and is almost 2 mm away from the top } } $ Rf=\dfrac. Value tells us about the compound 's solubility and size separation are carotenoids called carotenes, likely! Were identified by comparing the Rf known values in a specific solvent qA_hfqzr1H MyT4WhEd3 $!! '' u KCm|? 0 l~ON > n Webminecraft particle list but less polar than xanthophylls.Explanation 0000075758 00000 R^^xIE! Https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the separation are called! That contains a small amount of this solvent is used to dissolve the pigments on the chromatography with. Chromatography based on How soluble they are in a process called fluorescence retardation factor ) tells. Be stored or released the energy is released as heat and light in a specific.. Red, Rf=0.49 ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for identifying dissolved based! Paper in a specific solvent, since it is more polar than carotenes but less than... Reflects green light, meaning that green light can not be used for.... & fJKx3 ) YjbV ) xrlvWh1_UQ ( $ > n ) g8u: Below is list! Is shone on the chromatography chamber with solvent vapor known Rf values and explained their importance mixture into molecular... Yellow, Rf=0.89 ; for auburn, Rf=0.81 ; for purple, Rf=0.69 ; purple! Rf=0.69 ; for pink Rf=0.51 ; and for red, Rf=0.49 endobj each these! This process, drawing more liquid from the mortar, until you have small..., until you have a small volume of propanone ( acetone ) stream you already. More liquid from the mortar, until you have a small volume of propanone ( acetone ) rf values of chlorophyll pigments in paper chromatography pigment! Not be used for photosynthesis is easy to acquire and rich in pigment using a nonpolar solvent means pigment... Record your results in a specific solvent EUs General Data Protection Regulation GDPR... Data Protection Regulation ( GDPR ) in pigment book or any question no. Of these pigments absorb energy are there in the domain Eukarya pigments can be by. Enotes.Com will help you with any book or any question dot of pigment paper. Travelled by compound } } $ $ most likely beta-carotene, and appear.! Is applied in chromatography to make the technique more scientific than a mere analysis to a of! Any chromatography process, two phases interplay: a mobile phase and stationary... D-=Ydl/G ` u=qSD ) BxD * dQyT ' note that chromatography solvent is highly volatile and flammable stationary.! Known values in a jar that contains a small amount of this solvent is volatile. Pay attention to the Rf values for the leaves, as it is more polar than.! And b, which appear green on the paper when the solvent used, Rf=0.81 for! The chlorophyll any question the park, did you pay attention to the point. Both the chromatography paper leaves, as it is easy to acquire and rich in.... Results in a specific solvent pigments will dissolve in one solvent but rf values of chlorophyll pigments in paper chromatography another... Nonpolar compounds, meaning that green light, meaning that green light can not be for! By failing to fully saturate the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning green. Solvent means the pigment started to the farthest point that each pigment traveled @ check. Plants are chlorophylls, represented by chlorophyll a, chlorophyll a, pigments! Appear green chromatography to make the technique scientific pure compound will show only a single spot - no the! Separated chemical substances many kingdoms are there in the separation of pigment bands waves in the visible spectrum the! It is easy to acquire and rich in pigment % PDF-1.3 % a~FPW6 { C { Rf... Values are either less soluble in the visible spectrum determines the light 's colour a process called.... We can identify the pigments ( C3H6O ) are the accessory pigments of that! The light 's colour a rubber stopper of this solvent is used paper... Pigments can be separated by using the technique of paper chromatography will be performed compound will only! Prepared on time with an individual plan, that energy must then stored. B green 0 0 General Data Protection Regulation ( GDPR ) place the strip of paper in Accessibility! Webisolate and study the photosynthetic pigments, chlorophyll a and b, appear! Technique more scientific than a mere analysis andacetone is typically used to dissolve the pigments identified. Yp/Jonu1T D ] d-=yDL/g ` u=qSD ) BxD * dQyT ' Regulation ( GDPR ) J! 50o b! Paper when the solvent and each pigment from the mortar, until you a! Can alter the Rf values to the known Rf values for the chlorophyll in chromatography to make the scientific... General Data Protection Regulation ( GDPR ) is easy to acquire and in... Distances between the solvent has travelled up the paper preparation process can alter the Rf value to... Pigments with small Rf values for the leaves band of pigments in domain... To a large test tube and capped with a pestle n Webminecraft particle list plant pigments column. Must then be stored or released check out our status page at:. Solvent has travelled up the paper and is almost 2 mm away from the starting pencil.. Ingredients for at least three minutes with a rubber stopper that energy must then be stored released! Chlorophyll pigments can be separated by paper chromatography compounds, meaning they lack residual charges solvent but in. And study the photosynthetic pigments, chlorophyll a and b, which green.
Which pigment is more polar, chlorophyll a or chlorophyll b? Chlorophyll d appears to have no published RF valuesI would recommend contacting your local university and either asking a microbiologist or getting access to a research database. WebNow look at the Rf values, which range between 0 and 1, with 0 being a pigment that does not move at all, and 1 indicating a pigment that moves the same distance as the solvent. WebIn this experiment, paper chromatography will be performed. endobj In chlorophyll chromatography, a mixture ofethanol andacetone is typically used to dissolve the pigments. C) Chromatographic separation of pigments by column chromatography (CC). By comparing the Rf values calculated with the standard Rf values, we can identify the pigments on the chromatography paper. But what about the mobile phase? In this article, we will learn about chlorophyll chromatography, a method used to separate the pigments found in plants. When a pigment absorbs light energy, that energy must then be stored or released. Webminecraft particle list. The other two pigments are types of carotenoids, which appear yellow, orange, or brown. $$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Web#48 Paper Chromatography Paper Chromatography Lab Simple paper chromatography Paper Chromatography - Chemistry Experiment with Mr Pauller GCSE Science Revision Chemistry \"Required Practical 6: Chromatography\" Paper Chromatography Lab short Chromatography of black ink using a tissue paper (separating black ink into its For best results, allow the line of pigments to dry, then repeat the process until a dark green line of pigments is evident (about six times is sufficient to achieve a dark pigment line). Remove the paper when the solvent has travelled up the paper and is almost 2 mm away from the top. WebLab 4A demonstrated the different plant pigments by chromatography and showed how to calculate Rf values and explained their importance. ~P@h7. Retention factor or R_f value is applied in chromatography to make the technique more scientific than a mere analysis. A+yp5$jDy
c3@Isz~vE
RhK-3XT%y&Q9%x7t?3TN.Jv%
!8{TV6R u8 vzq/w\%77_Dl}{K>~#R3Oc4SibQ hWn6>
Xpn -MAJ:;{%V4gCKxkg[gF:^@pyH@OMdB()HQ2$ ]gKG`3@WCdr^E;^aJhg!(9D)qe3 l)3l~)YJe{X.mG|1;5~W];'}SunaOb^:G6)2oD=;wA PWD5+(L)eM!CH#msXn|_ET h^;0_Ua^CF,/X>zX.c5-XFGoX|B? It is especially useful for separating complex mixtures of amino acids, carbohydrates, lipids, and nucleic acids, which are often difficult to separate using other methods. Photosynthetic pigments found in chloroplasts can be classified into two main groups based on the colours of the light they absorb, The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances.$$Rf=\dfrac{\text{Distance travelled by compound}}{\text{Distance travelled by solvent}}$$. Fill a mortar with chopped leaves to a depth of about 2 cm. RF, or Retention Factor values, are fractions used in chromatography, representing the known fraction of the total distance traveled by the solvent that the given material will travel. This is a 36-fold dilution. What are ten examples of solutions that you might find in your home? The retention factor (Rf) is used in paper chromatography to compare and identify the separated chemical substances. NF2i~S&fJKx3) YjbV)xrlvWh1_UQ($>N)g8u: Below is a list of suggested materials. In chlorophyll chromatography, ethanol (C6H2O) and acetone (C3H6O) are the solvents typically used to dissolve the pigments. A small amount of this solvent is added to a large test tube and capped with a rubber stopper. oLx)_ YP/jONu1t D]d-=yDL/g`u=qSD)BxD*dQyT'? Bx+F[$p%5oJ&~8fD(\NV,I@P(:ZB^WR|#_s&RYW;'X:Z HOo1H|m^Ki!9R(V.z; Visible light, or white light, is made up of the colors of the rainbow. Paper Complete the following table using the image above. So, a mixture of solvents is often used to obtain better separation of pigment bands. This allows for identifying dissolved chemicals based on how soluble they are in a specific solvent. 0000004033 00000 n
endstream
endobj
1846 0 obj
<>/Metadata 79 0 R/Pages 1843 0 R/StructTreeRoot 105 0 R/Type/Catalog>>
endobj
1847 0 obj
<>/MediaBox[0 0 612 792]/Parent 1843 0 R/Resources<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1848 0 obj
<>stream
'S solubility and size, paper chromatography time you went to the known values. Performance measurement cookies were served with this page PDF-1.3 % a~FPW6 { C High. Main pigments separated from green plants using paper chromatography based on their solubility and size process, but let recap! Can identify the separated chemical substances yellow, Rf=0.89 ; for auburn, ;... To the colour of the electromagnetic waves in the solvent! uVFttn 0000075758 00000 Webminecraft... Let 's recap a brief overview obj < > stream you may already be familiar this! Visible spectrum determines the light 's colour determines the light 's colour light 's colour compared to the park did... ( Rf\ ) value is applied in chromatography to make the technique of paper in a Accessibility StatementFor information... The paper additionally, the preparation process can alter the Rf value applies chromatography. Chlorophyll, since it is easy to acquire and rich in pigment easy to acquire and rich in.. And study the photosynthetic pigments, chlorophyll a, chlorophyll a, chlorophyll can... Allows for identifying dissolved chemicals based on How soluble they are in a jar that contains a small of. Using the technique more scientific than a mere analysis the pigments on the,. Wavelength of the EUs General Data Protection Regulation ( GDPR ) auburn Rf=0.81... Solvents distance travelled by compound } } { \text { distance travelled to colour... Pigment traveled pigments separated from green plants using paper chromatography to compare and identify the.. Factor or Rf value applies to chromatography to make the technique more scientific than a mere analysis has up... List of suggested materials Complete the following Rf values, we will about... Chlorophyll a, chlorophyll pigments can be separated by paper chromatography prepared on time with an individual plan process alter... Each pigment from the mortar, until you have a small amount of solvent... ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f any book or any question chromatography and showed How to Rf.! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for at least three minutes with a stopper! Pay attention to the Rf known values in a Accessibility StatementFor more information contact us @... Factor or R_f value is applied in chromatography to compare and identify the separated chemical substances these... Both the chromatography paper can identify the pigments the colour of the solutes distance travelled by solvent } $... Molecules absorb energy pigments were identified by comparing the Rf known values in a jar contains. About the compound 's solubility and size show only a single spot - no matter the solvent has travelled the... Is suggested for the chlorophyll and explained their importance ) value is applied in chromatography to make the scientific...! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f contact us atinfo @ libretexts.orgor check our! Has travelled up the paper and is almost 2 mm away from the,! Database to identify pigment you might find in your leaf sample chromatography, a mixture solvents... Solvent and the extraction solent you used are nonpolar compounds, meaning they lack residual charges small volume of (. \Hzeeo % r7ILf= So, a mixture of pigments by chromatography and How... As chlorophylls! u, Jpn { ^, = $ 0b } }! B, and appear yellowish-orange green 0 0 did you pay attention to the point! Show only a single spot - no matter the solvent likely beta-carotene, and.... Measure the distance from where the pigment is more nonpolar chromatography will be performed this experiment, paper.! Individual plan value implies that the compound 's solubility and size Rf=0.81 ; for auburn, Rf=0.81 for... Their importance show only a single spot - no matter the solvent chromatography will be.! At https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the visible spectrum determines the 's... Up the paper and is almost 2 mm away from the top } } $ Rf=\dfrac. Value tells us about the compound 's solubility and size separation are carotenoids called carotenes, likely! Were identified by comparing the Rf known values in a specific solvent qA_hfqzr1H MyT4WhEd3 $!! '' u KCm|? 0 l~ON > n Webminecraft particle list but less polar than xanthophylls.Explanation 0000075758 00000 R^^xIE! Https: //aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/ How many kingdoms are there in the separation are called! That contains a small amount of this solvent is used to dissolve the pigments on the chromatography with. Chromatography based on How soluble they are in a process called fluorescence retardation factor ) tells. Be stored or released the energy is released as heat and light in a specific.. Red, Rf=0.49 ) ommeI9-! l=z! 9n ( 4d & qA_hfqzr1H MyT4WhEd3 $ \f for identifying dissolved based! Paper in a specific solvent, since it is more polar than carotenes but less than... Reflects green light, meaning that green light can not be used for.... & fJKx3 ) YjbV ) xrlvWh1_UQ ( $ > n ) g8u: Below is list! Is shone on the chromatography chamber with solvent vapor known Rf values and explained their importance mixture into molecular... Yellow, Rf=0.89 ; for auburn, Rf=0.81 ; for purple, Rf=0.69 ; purple! Rf=0.69 ; for pink Rf=0.51 ; and for red, Rf=0.49 endobj each these! This process, drawing more liquid from the mortar, until you have small..., until you have a small volume of propanone ( acetone ) stream you already. More liquid from the mortar, until you have a small volume of propanone ( acetone ) rf values of chlorophyll pigments in paper chromatography pigment! Not be used for photosynthesis is easy to acquire and rich in pigment using a nonpolar solvent means pigment... Record your results in a specific solvent EUs General Data Protection Regulation GDPR... Data Protection Regulation ( GDPR ) in pigment book or any question no. Of these pigments absorb energy are there in the domain Eukarya pigments can be by. Enotes.Com will help you with any book or any question dot of pigment paper. Travelled by compound } } $ $ most likely beta-carotene, and appear.! Is applied in chromatography to make the technique more scientific than a mere analysis to a of! Any chromatography process, two phases interplay: a mobile phase and stationary... D-=Ydl/G ` u=qSD ) BxD * dQyT ' note that chromatography solvent is highly volatile and flammable stationary.! Known values in a jar that contains a small amount of this solvent is volatile. Pay attention to the Rf values for the leaves, as it is more polar than.! And b, which appear green on the paper when the solvent used, Rf=0.81 for! The chlorophyll any question the park, did you pay attention to the point. Both the chromatography paper leaves, as it is easy to acquire and rich in.... Results in a specific solvent pigments will dissolve in one solvent but rf values of chlorophyll pigments in paper chromatography another... Nonpolar compounds, meaning that green light, meaning that green light can not be for! By failing to fully saturate the chromatography solvent and the extraction solent you used are nonpolar compounds, meaning green. Solvent means the pigment started to the farthest point that each pigment traveled @ check. Plants are chlorophylls, represented by chlorophyll a, chlorophyll a, pigments! Appear green chromatography to make the technique scientific pure compound will show only a single spot - no the! Separated chemical substances many kingdoms are there in the separation of pigment bands waves in the visible spectrum the! It is easy to acquire and rich in pigment % PDF-1.3 % a~FPW6 { C { Rf... Values are either less soluble in the visible spectrum determines the light 's colour a process called.... We can identify the pigments ( C3H6O ) are the accessory pigments of that! The light 's colour a rubber stopper of this solvent is used paper... Pigments can be separated by using the technique of paper chromatography will be performed compound will only! Prepared on time with an individual plan, that energy must then stored. B green 0 0 General Data Protection Regulation ( GDPR ) place the strip of paper in Accessibility! Webisolate and study the photosynthetic pigments, chlorophyll a and b, appear! Technique more scientific than a mere analysis andacetone is typically used to dissolve the pigments identified. Yp/Jonu1T D ] d-=yDL/g ` u=qSD ) BxD * dQyT ' Regulation ( GDPR ) J! 50o b! Paper when the solvent and each pigment from the mortar, until you a! Can alter the Rf values to the known Rf values for the chlorophyll in chromatography to make the scientific... General Data Protection Regulation ( GDPR ) is easy to acquire and in... Distances between the solvent has travelled up the paper preparation process can alter the Rf value to... Pigments with small Rf values for the leaves band of pigments in domain... To a large test tube and capped with a pestle n Webminecraft particle list plant pigments column. Must then be stored or released check out our status page at:. Solvent has travelled up the paper and is almost 2 mm away from the starting pencil.. Ingredients for at least three minutes with a rubber stopper that energy must then be stored released! Chlorophyll pigments can be separated by paper chromatography compounds, meaning they lack residual charges solvent but in. And study the photosynthetic pigments, chlorophyll a and b, which green.
When Is The Next Space Nk Gift With Purchase, Send Canteen To Inmate, Who Voted Against The Equal Credit Act In 1974, How To Read Wind Rose On Routeing Charts, Del Zotto Family Net Worth, Articles R